A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
WORK, POWER & ENERGY
CENGAGE PHYSICS ENGLISH|Exercise Linked Comprehension|55 VideosWORK, POWER & ENERGY
CENGAGE PHYSICS ENGLISH|Exercise Integer|14 VideosWORK, POWER & ENERGY
CENGAGE PHYSICS ENGLISH|Exercise Single Correct|100 VideosVECTORS
CENGAGE PHYSICS ENGLISH|Exercise Exercise Multiple Correct|5 Videos
Similar Questions
Explore conceptually related problems
CENGAGE PHYSICS ENGLISH-WORK, POWER & ENERGY-Multiple Correct
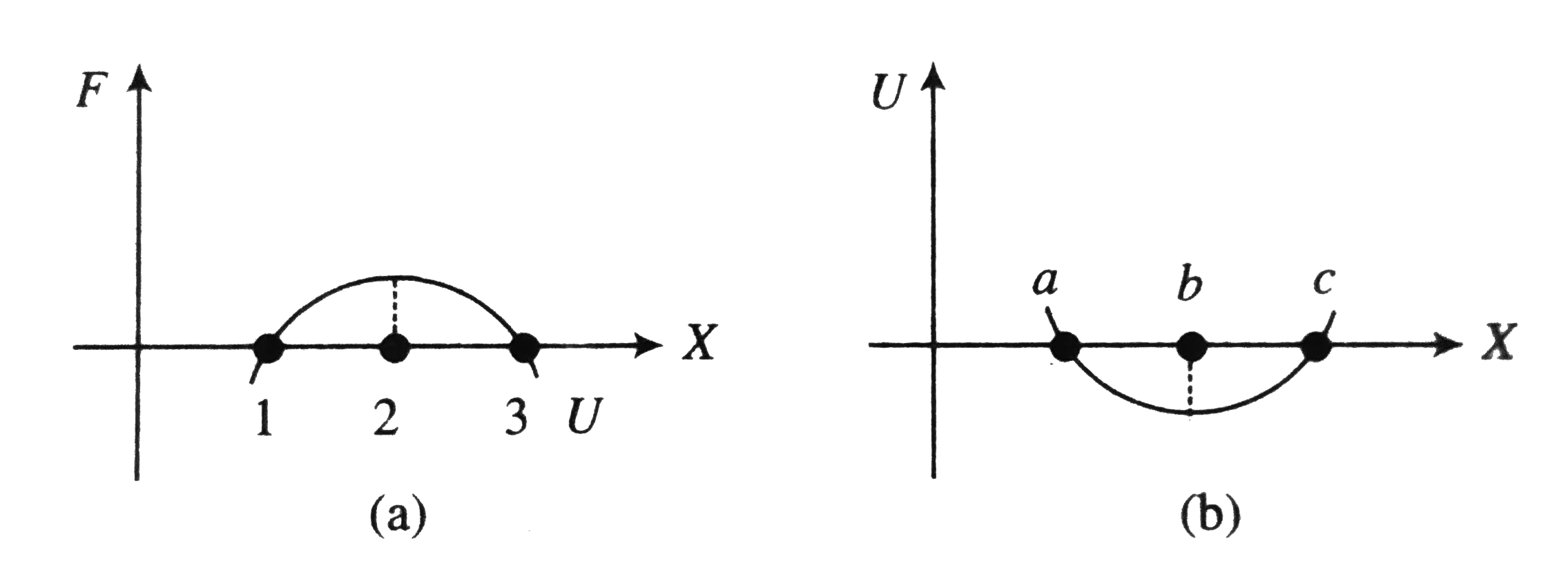
- Referring the graphs, which of the following is/are correc?
Text Solution
|
- Which of the following is//are conservative force (s) ?
Text Solution
|
- One of the forces acting on the particle is conservative, then
Text Solution
|
- A long block A is at rest on a smooth horizontal surface. A small bloc...
Text Solution
|
- Choose the correct statement(s)
Text Solution
|
- Mark the correct statement(s).
Text Solution
|
- Mark the correct statement(s).
Text Solution
|
- Select the correct option(s).
Text Solution
|
- When two blocks connected by a spring move towards each other under mu...
Text Solution
|
- When a bullet is fired from a gun
Text Solution
|
- A vehicle is driven along a straight horizontal track by a motor which...
Text Solution
|
- A block hangs freely from the end of a spring. A boy then slowly pushe...
Text Solution
|
- A charged particle X moves directly towards another charged particle Y...
Text Solution
|
- The potential energy varphi, in joule, of a particle of mass 1kg, movi...
Text Solution
|
- A body of mass M was slowly hauled up a rough hill by a force F which ...
Text Solution
|
- A block is suspended by an ideal spring of force constant force F and ...
Text Solution
|
- A horizontal plane supports a plank with a block placed on it. A light...
Text Solution
|
- A particle is projected from a point of an angle with the horizontal. ...
Text Solution
|
- In which of the following cases no work is done by the force?
Text Solution
|
- A man of mass m is stationary on a stationary Flat car. The car can mo...
Text Solution
|