Text Solution
Verified by Experts
Topper's Solved these Questions
PHOTOELECTRIC EFFECT
CENGAGE PHYSICS ENGLISH|Exercise Exercise 3.2|8 VideosPHOTOELECTRIC EFFECT
CENGAGE PHYSICS ENGLISH|Exercise Subjective|16 VideosPHOTOELECTRIC EFFECT
CENGAGE PHYSICS ENGLISH|Exercise Examples|8 VideosNUCLEAR PHYSICS
CENGAGE PHYSICS ENGLISH|Exercise ddp.5.5|14 VideosRAY OPTICS
CENGAGE PHYSICS ENGLISH|Exercise DPP 1.6|12 Videos
Similar Questions
Explore conceptually related problems
CENGAGE PHYSICS ENGLISH-PHOTOELECTRIC EFFECT-Exercise 3.1
- Find the de Broglie wavelength of 2 MeV proton. Mass of proton =1.64xx...
Text Solution
|
- Find the de Broglie wavlength of a neutron at 127^circC Given that Bol...
Text Solution
|
- The intensity of direct sunlight before it passes through the earth...
Text Solution
|
- An electron is accelerated by a potential difference of 25 V. Find the...
Text Solution
|
- Why are de Broglie waves associated with a moving football is not appa...
Text Solution
|
- The de Broglie wavelength of a particel of kinetic energy K is lamda. ...
Text Solution
|
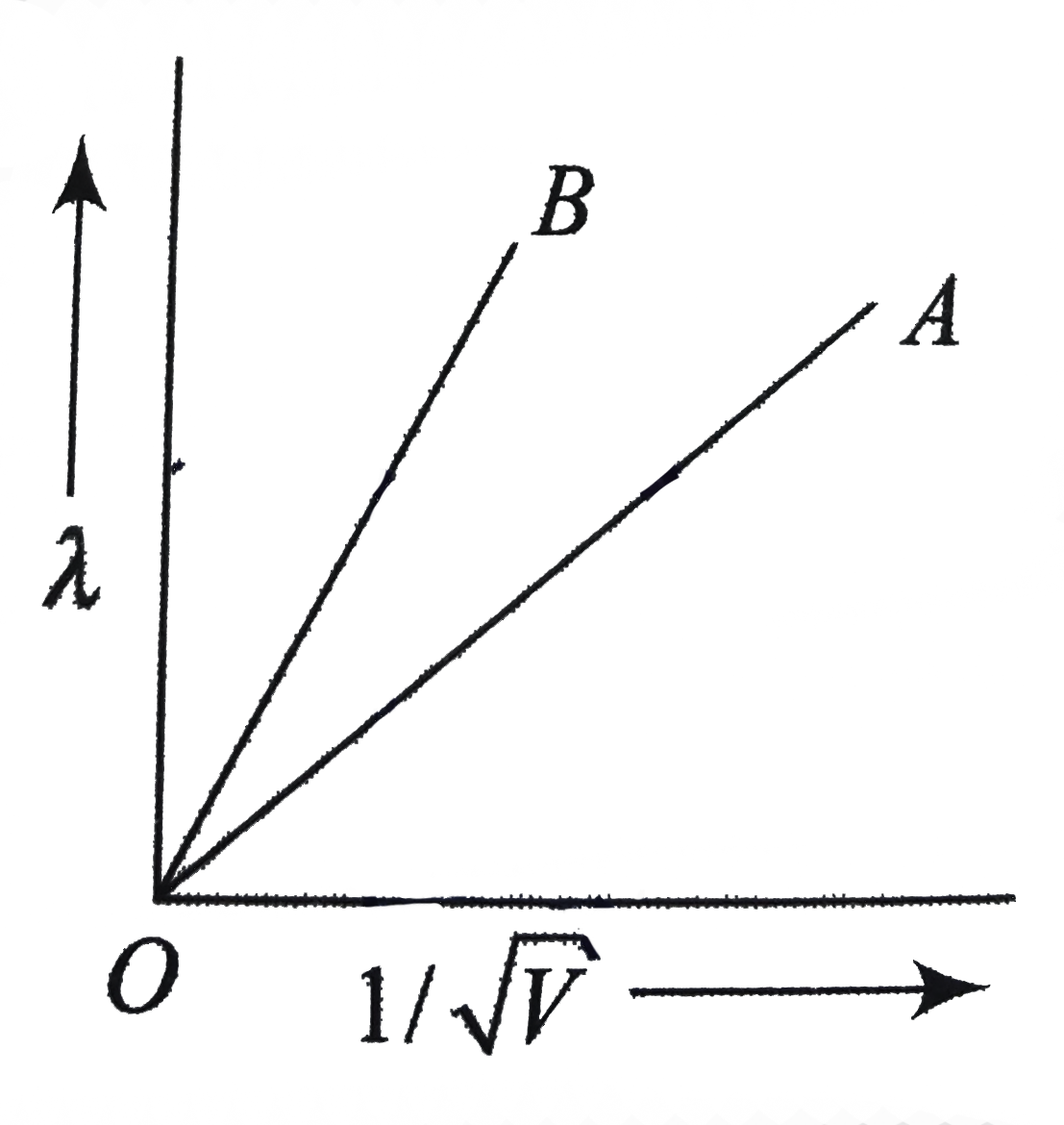
- The two lines A and B in fig. shows the plot of de- Broglie wavelength...
Text Solution
|
- A helium-neon laser emits light of wavelength 632.8nm. Calculate the e...
Text Solution
|
- The energy flux of sunlight reaching the surface of the earth is 1.388...
Text Solution
|
- An electron and a photon each have a wavelength of 1.00 nm. Find (a) t...
Text Solution
|
- For a given kinetic energy, which of the following has the smallest de...
Text Solution
|
- A plate is kept in front of a beam of photons. The plate reflects 40% ...
Text Solution
|
- A sensor is exposed for 0.1 s to a 200 W lamp 10m away The sensor has ...
Text Solution
|
- Photons of wavelength lamda=662 nm are incident normally on a perfectl...
Text Solution
|
- A voltage applied to an X-ray tube being increased eta = 1.5 times, t...
Text Solution
|