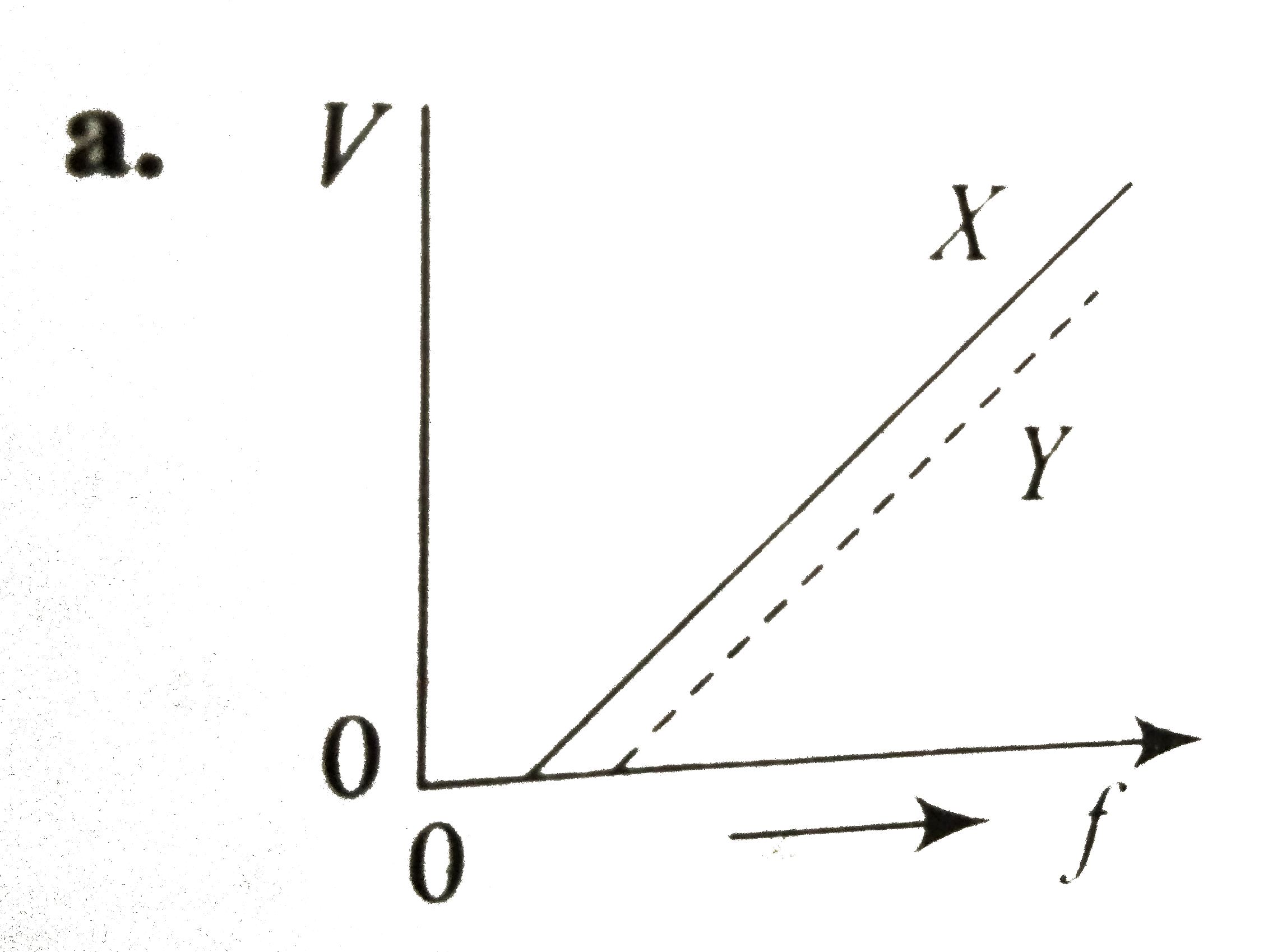
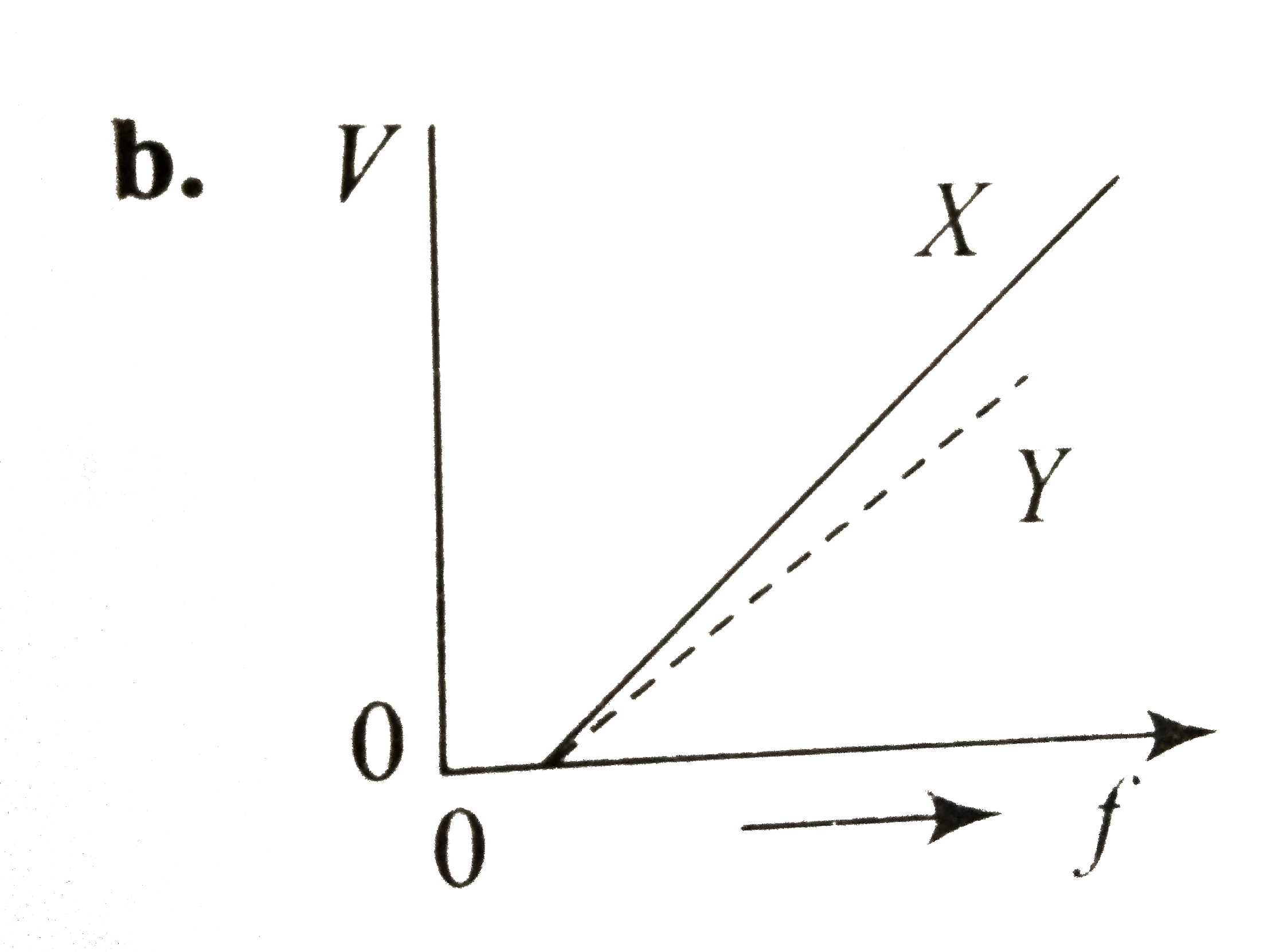
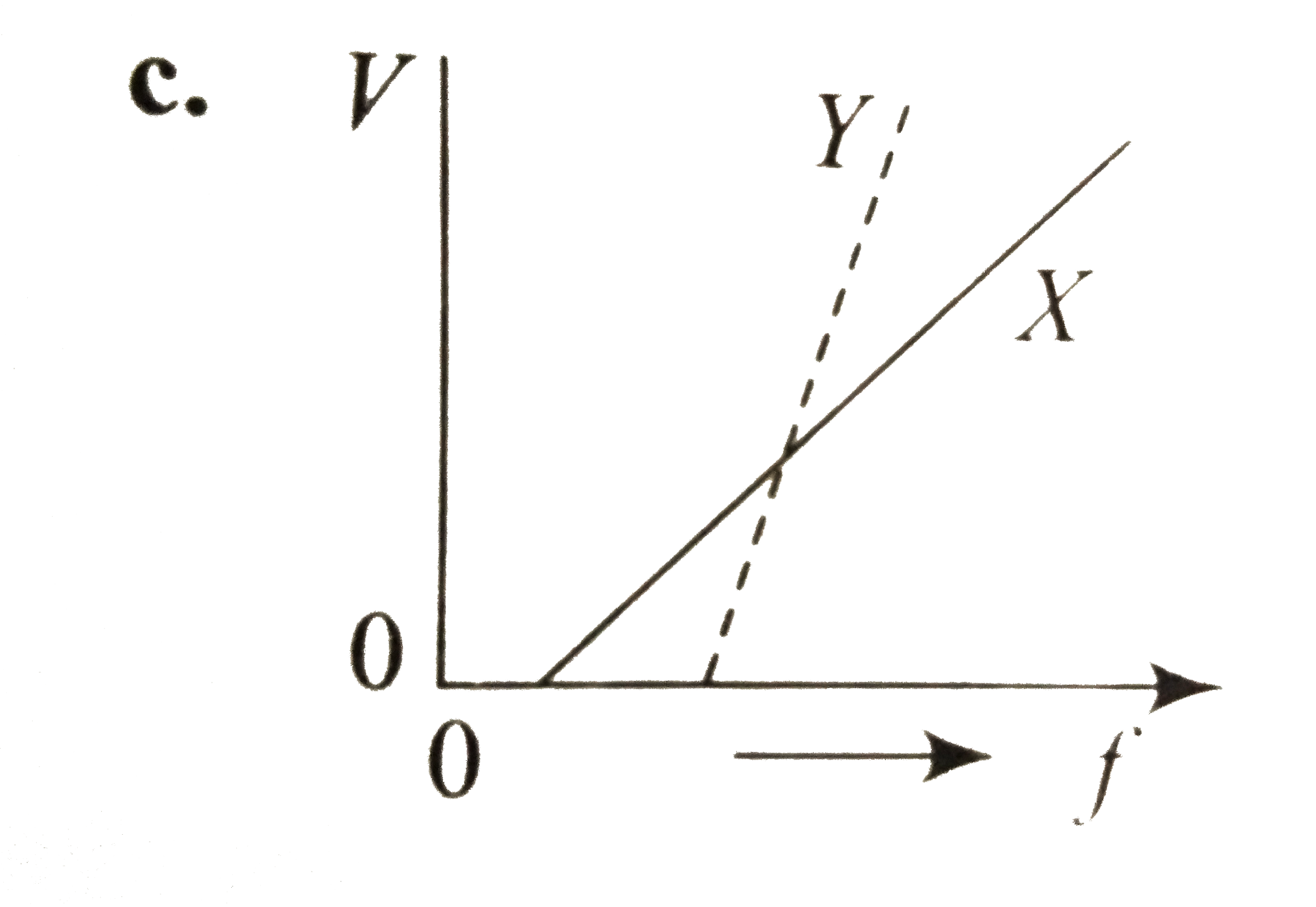
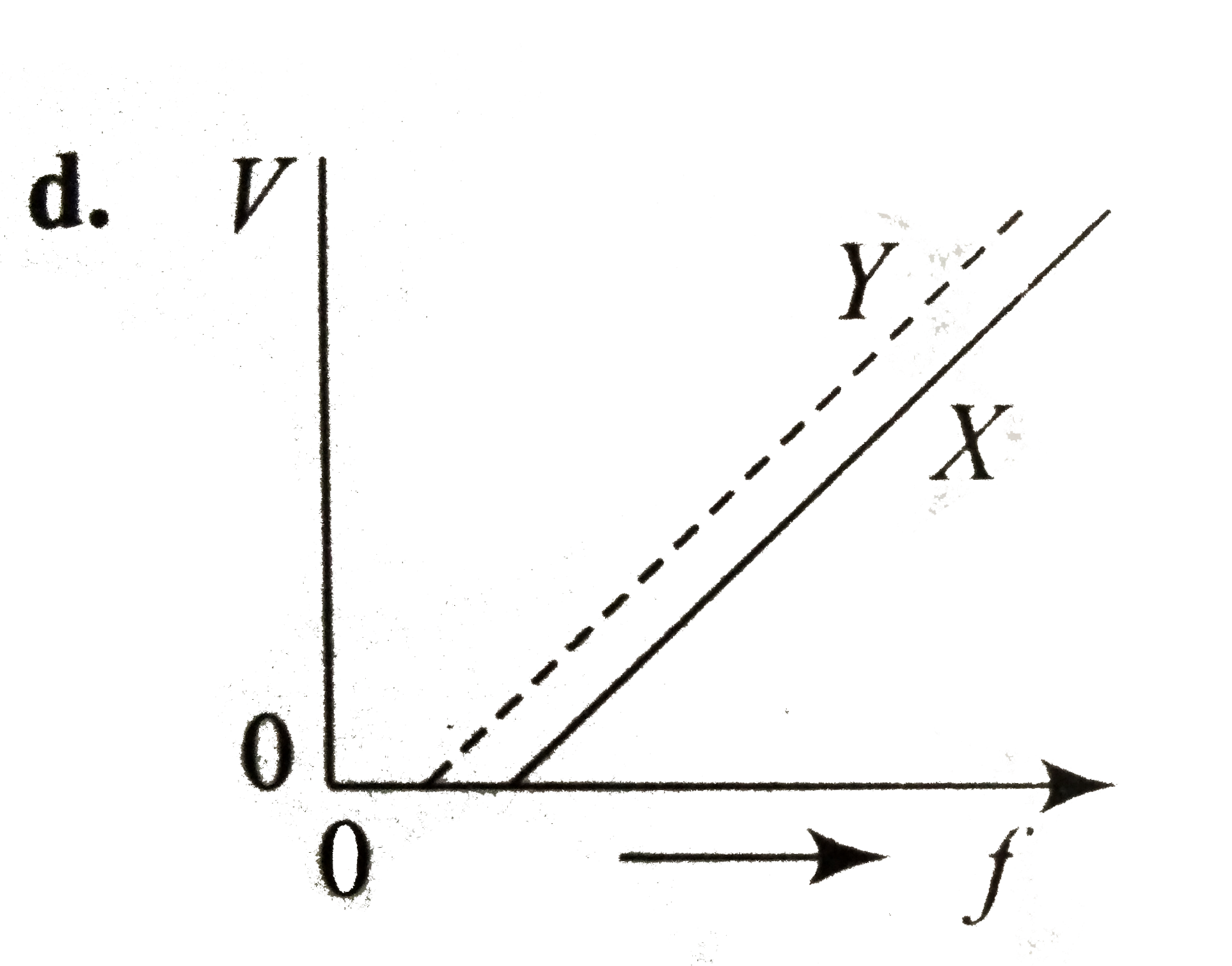A
B
C
D
Text Solution
AI Generated Solution
The correct Answer is:
Topper's Solved these Questions
PHOTOELECTRIC EFFECT
CENGAGE PHYSICS ENGLISH|Exercise Multiple Correct|10 VideosPHOTOELECTRIC EFFECT
CENGAGE PHYSICS ENGLISH|Exercise Linked Comprehension|44 VideosPHOTOELECTRIC EFFECT
CENGAGE PHYSICS ENGLISH|Exercise Subjective|16 VideosNUCLEAR PHYSICS
CENGAGE PHYSICS ENGLISH|Exercise ddp.5.5|14 VideosRAY OPTICS
CENGAGE PHYSICS ENGLISH|Exercise DPP 1.6|12 Videos
Similar Questions
Explore conceptually related problems
CENGAGE PHYSICS ENGLISH-PHOTOELECTRIC EFFECT-Single Correct
- If lamda0 stands for mid-wavelength in the visible region, the de Brog...
Text Solution
|
- The threshold frequency for certain metal is v0. When light of frequen...
Text Solution
|
- In a photoelectric emission, electrons are ejected from metals X and Y...
Text Solution
|
- In a photoelectric cell, the wavelength of incident light is chaged fr...
Text Solution
|
- When a metallic surface is illuminated by a light of frequency 8xx10^(...
Text Solution
|
- The de Broglie wavelength of neutrons in thermal equilibrium is (given...
Text Solution
|
- A partical of mass M at rest decays into two Particles of masses m1 ...
Text Solution
|
- What is the energy of a proton possessing wavelength 0.4A?
Text Solution
|
- An electron and a photon possess the same de Broglie wavelength. If Ee...
Text Solution
|
- In Q.72,An electron and a photon possess the same de Broglie wavelengt...
Text Solution
|
- An electron and a photon, each has a wavelength of 1.2A. What is the r...
Text Solution
|
- What is the wavelength of a photon of energy 1 eV?
Text Solution
|
- If lamda1 and lamda2 denote the wavelength of de Broglie waves for ele...
Text Solution
|
- Which curve shows the relation ship between the energy R and the wavel...
Text Solution
|
- Work function of nickel is 5.01 eV. When ultraviolet radiation of wave...
Text Solution
|
- An electron is accelerated through a potential difference of V volt. I...
Text Solution
|
- The kinetic energy of most energetic electrons emitted from a metallic...
Text Solution
|
- How many photons are emitted per second by a 5 m W laser source operat...
Text Solution
|
- Figure is the plot of the stopping potential versus the frequency of t...
Text Solution
|
- Figure is the plot of the stopping potential versus the frequency of t...
Text Solution
|