A
B
C
D
Text Solution
AI Generated Solution
The correct Answer is:
Topper's Solved these Questions
PHOTOELECTRIC EFFECT
CENGAGE PHYSICS ENGLISH|Exercise Multiple Correct|10 VideosPHOTOELECTRIC EFFECT
CENGAGE PHYSICS ENGLISH|Exercise Linked Comprehension|44 VideosPHOTOELECTRIC EFFECT
CENGAGE PHYSICS ENGLISH|Exercise Subjective|16 VideosNUCLEAR PHYSICS
CENGAGE PHYSICS ENGLISH|Exercise ddp.5.5|14 VideosRAY OPTICS
CENGAGE PHYSICS ENGLISH|Exercise DPP 1.6|12 Videos
Similar Questions
Explore conceptually related problems
CENGAGE PHYSICS ENGLISH-PHOTOELECTRIC EFFECT-Single Correct
- What is the wavelength of a photon of energy 1 eV?
Text Solution
|
- If lamda1 and lamda2 denote the wavelength of de Broglie waves for ele...
Text Solution
|
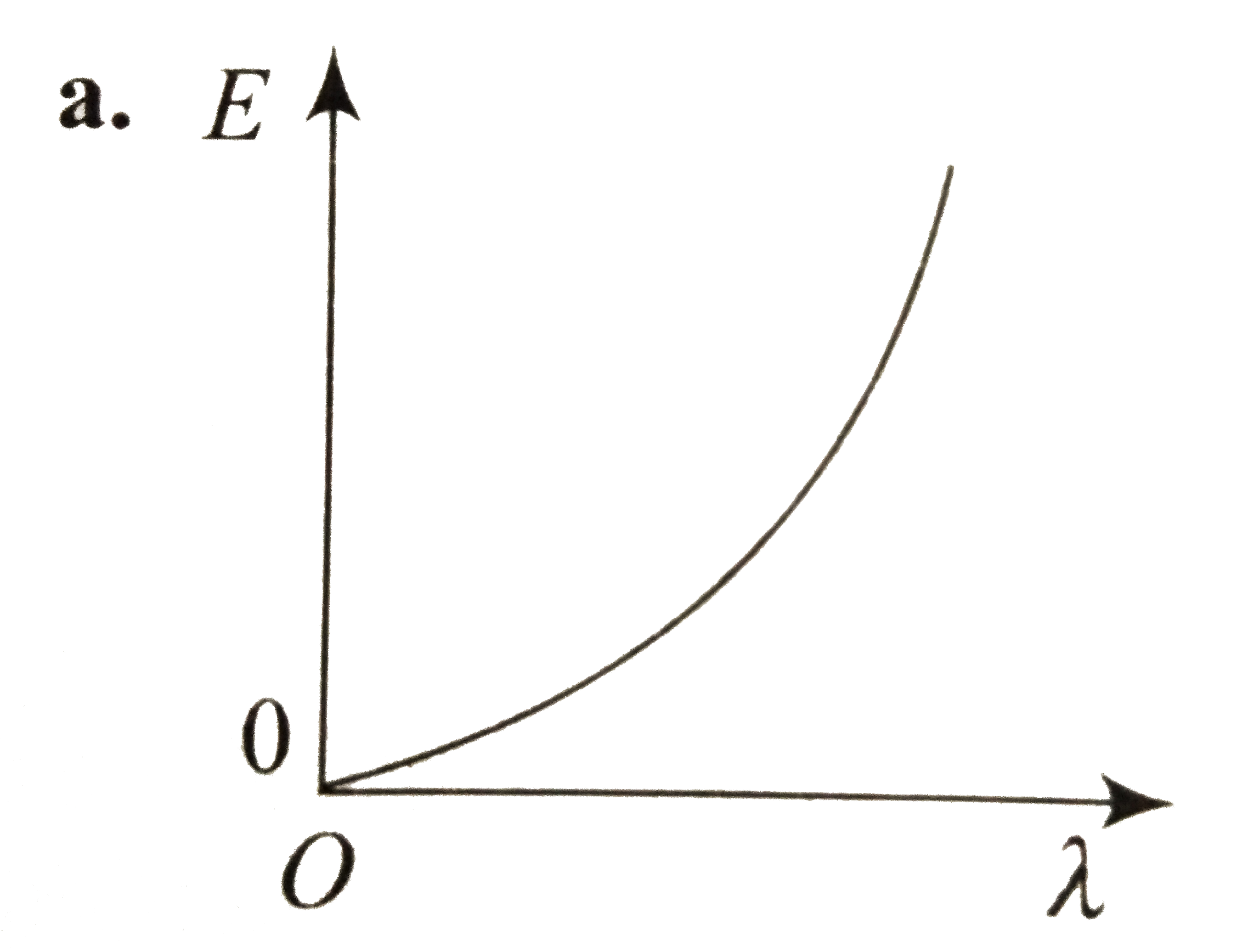
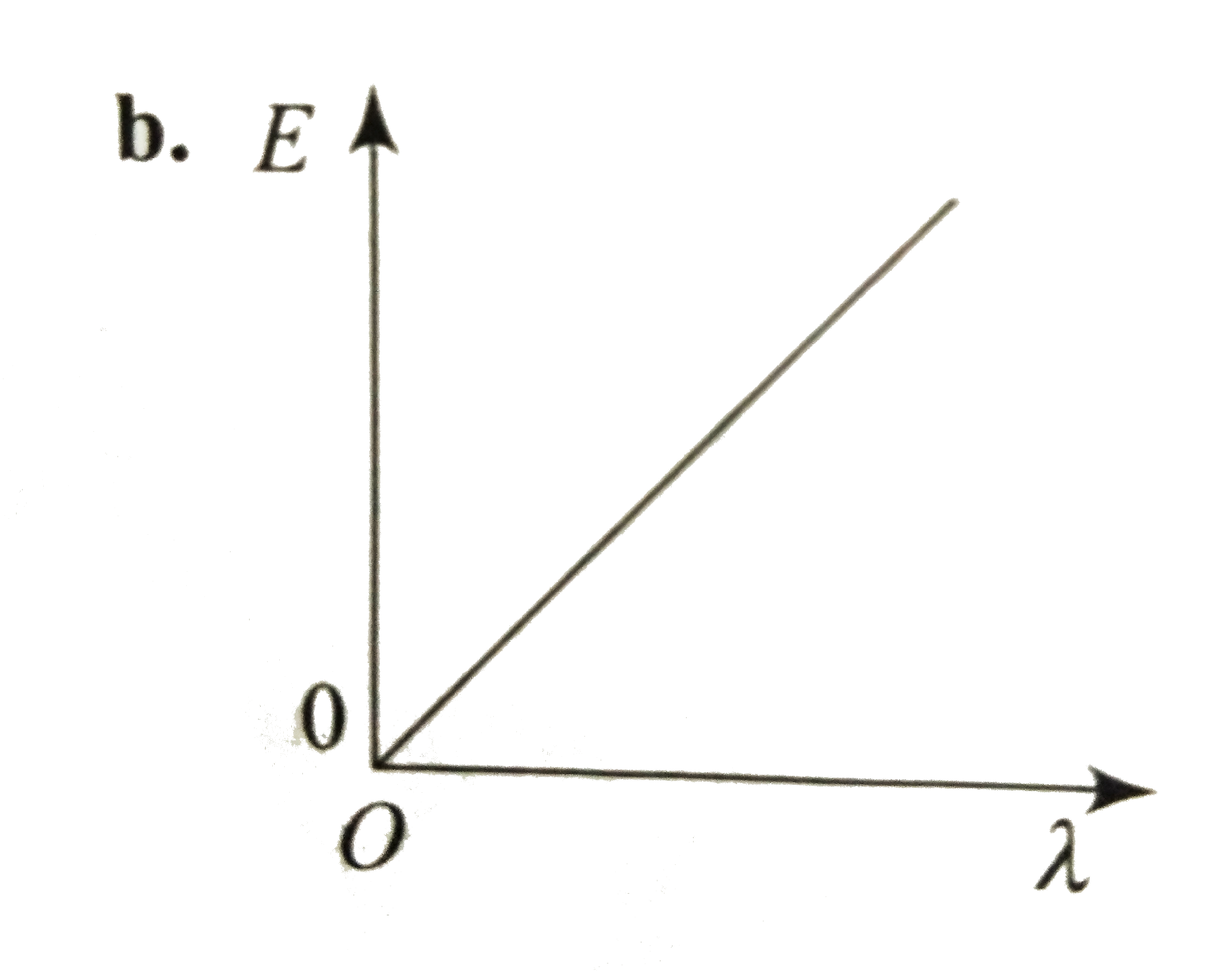
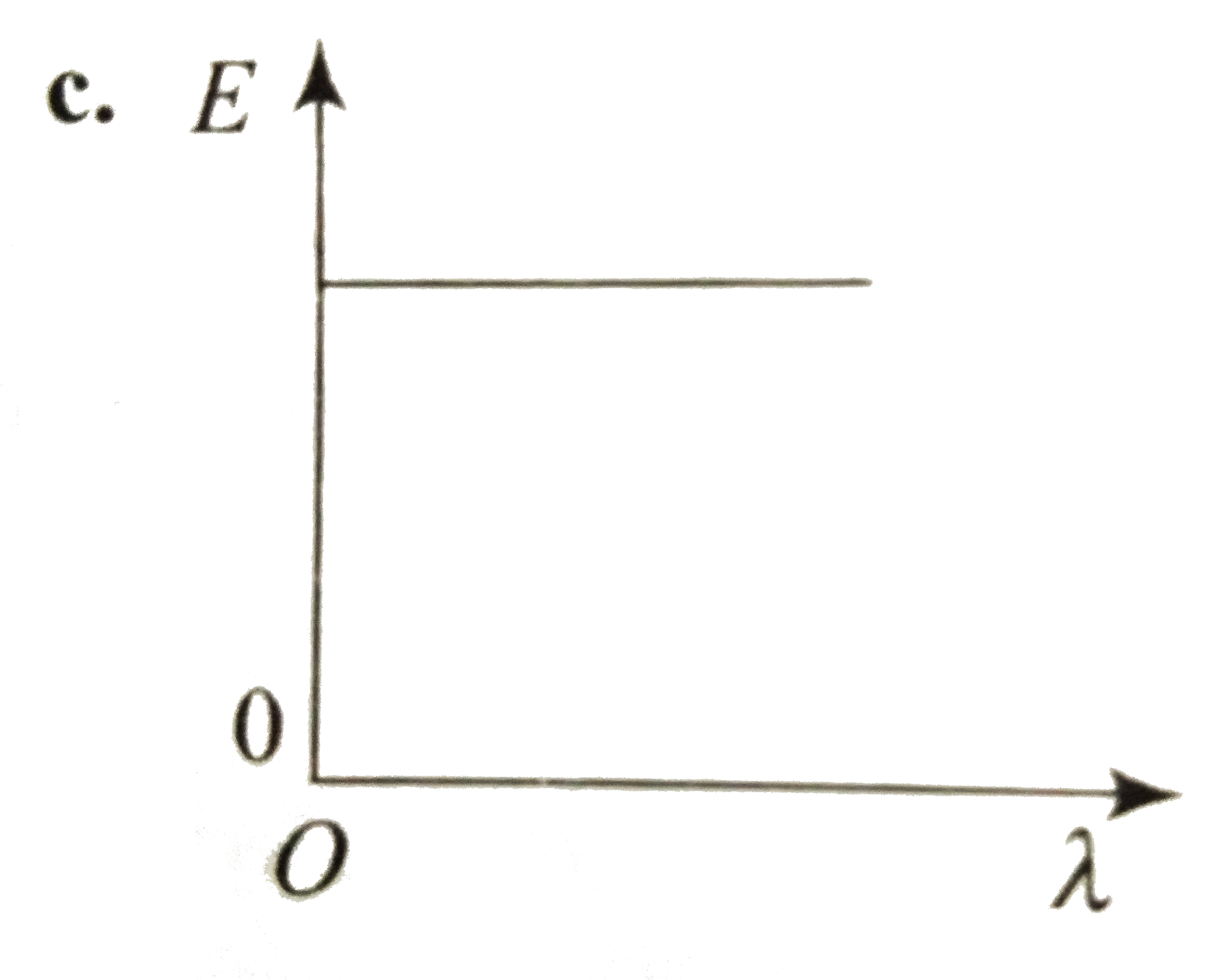
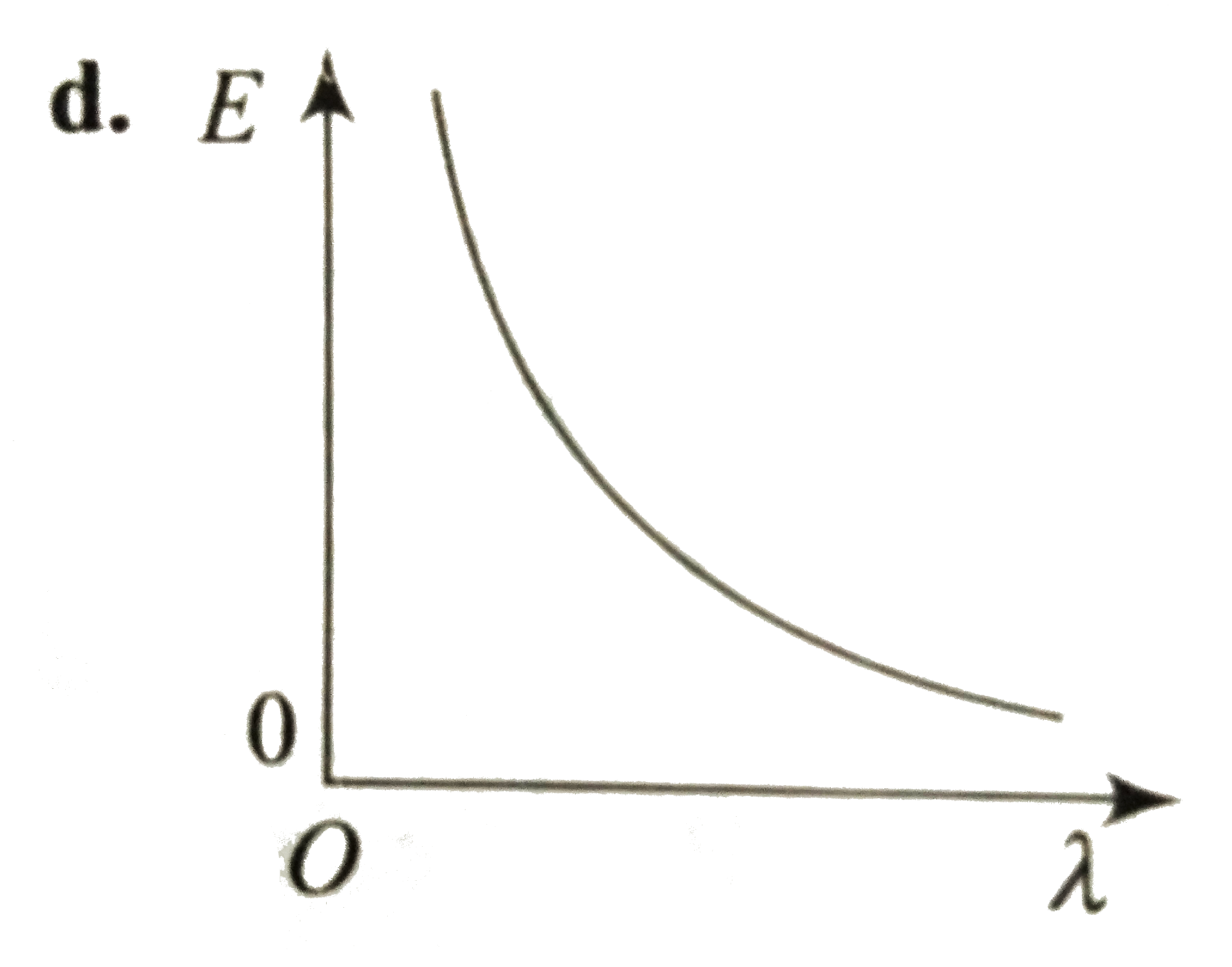
- Which curve shows the relation ship between the energy R and the wavel...
Text Solution
|
- Work function of nickel is 5.01 eV. When ultraviolet radiation of wave...
Text Solution
|
- An electron is accelerated through a potential difference of V volt. I...
Text Solution
|
- The kinetic energy of most energetic electrons emitted from a metallic...
Text Solution
|
- How many photons are emitted per second by a 5 m W laser source operat...
Text Solution
|
- Figure is the plot of the stopping potential versus the frequency of t...
Text Solution
|
- Figure is the plot of the stopping potential versus the frequency of t...
Text Solution
|
- Two identical metal plates show photoelectric effect. Light of wavelen...
Text Solution
|
- The potential difference applied to an X-ray tube is V The ratio of th...
Text Solution
|
- The maximum velocity of the photoelectrons emitted from the surface is...
Text Solution
|
- A homogeneous ball (mass=m) of ideal black material at rest is illumin...
Text Solution
|
- The eye can detect 5xx10^(4) photons (m^2s)^(-1) of green light (lamda...
Text Solution
|
- A photon of wavelength 0.1 A is emitted by a helium atom as a conseque...
Text Solution
|
- A monochromatic source of lightis placed at a large distance d From a ...
Text Solution
|
- A. How many photons of a tradiation of wavelength lamda=5xx10^(-7) m m...
Text Solution
|
- A nozzle throws a stream of gas against a wall with a velocity v much ...
Text Solution
|
- A plane light wave of intensity I=0.20Wcm^-2 falls on a plane mirror s...
Text Solution
|
- A plane wave of intensity I=0.70W cm^(-2) illuminates a sphere with id...
Text Solution
|