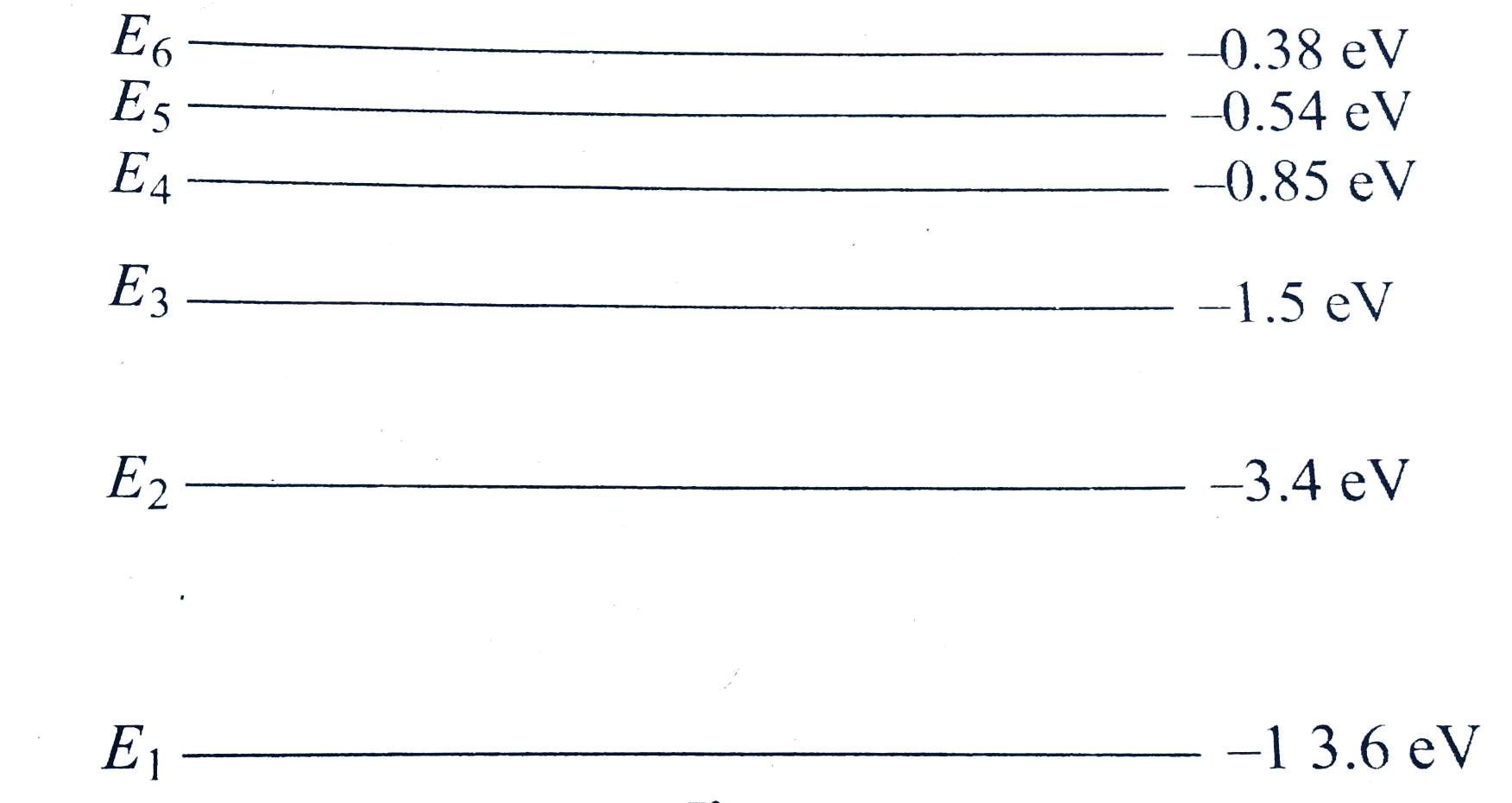
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
ATOMIC PHYSICS
CENGAGE PHYSICS ENGLISH|Exercise Multiple Correct|13 VideosATOMIC PHYSICS
CENGAGE PHYSICS ENGLISH|Exercise Linked Comprehension|62 VideosATOMIC PHYSICS
CENGAGE PHYSICS ENGLISH|Exercise Subject|17 VideosALTERNATING CURRENT
CENGAGE PHYSICS ENGLISH|Exercise Single Correct|10 VideosCAPACITOR AND CAPACITANCE
CENGAGE PHYSICS ENGLISH|Exercise Integer|5 Videos
Similar Questions
Explore conceptually related problems
CENGAGE PHYSICS ENGLISH-ATOMIC PHYSICS-Single Correct
- With the increase in peinciple quantum number, the energy difference b...
Text Solution
|
- If lambda(1) and lambda(2) are the wavelength of the first members of ...
Text Solution
|
- In figure E(1) and E(2) represent some of the energy levels of the hyd...
Text Solution
|
- Which of the following parameters are the same for all hydrogen like a...
Text Solution
|
- An atom emits a spectral line of wavelength lambda when an electron m...
Text Solution
|
- The frequency of emission line for eny transition in positronium atom...
Text Solution
|
- If the wavelength of photon emitted due to transition of electron from...
Text Solution
|
- Which of the following is true when Balmer gave his model for hydrogen...
Text Solution
|
- The shortest wavelength in the Lyman series of hydrogen spectrum is 91...
Text Solution
|
- The ratio of maximum to minimum possible radiation energy Bohr's hyp...
Text Solution
|
- A stationary hydrogen atom emits photon corresponding to the first lin...
Text Solution
|
- In interperting Rutherford's experiments on the scattering of alpha pa...
Text Solution
|
- An electron jumps from the fourth orbit to the second orbit hydrogen a...
Text Solution
|
- Given mass number of gold = 197, Density of gold = 19.7 g cm^(-3). The...
Text Solution
|
- Three energy levels of an atom are shown in figure . The wavelength co...
Text Solution
|
- The ratio of the speed of the electron in the first Bohr orbit of hydr...
Text Solution
|
- Suppose two deuterons must get as close as 10^(-14) m in order for the...
Text Solution
|
- An electron in H atom makes a transition from n = 3 to n = 1. The reco...
Text Solution
|
- A hydrogen-like atom emits radiation of frequency 2.7 xx 10^(15) Hz wh...
Text Solution
|
- An electron in a hydrogen atom makes a trsnsition n(1)rarrn(2) where n...
Text Solution
|