A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
ATOMIC PHYSICS
CENGAGE PHYSICS ENGLISH|Exercise Multiple Correct|13 VideosATOMIC PHYSICS
CENGAGE PHYSICS ENGLISH|Exercise Linked Comprehension|62 VideosATOMIC PHYSICS
CENGAGE PHYSICS ENGLISH|Exercise Subject|17 VideosALTERNATING CURRENT
CENGAGE PHYSICS ENGLISH|Exercise Single Correct|10 VideosCAPACITOR AND CAPACITANCE
CENGAGE PHYSICS ENGLISH|Exercise Integer|5 Videos
Similar Questions
Explore conceptually related problems
CENGAGE PHYSICS ENGLISH-ATOMIC PHYSICS-Single Correct
- An electron jumps from the fourth orbit to the second orbit hydrogen a...
Text Solution
|
- Given mass number of gold = 197, Density of gold = 19.7 g cm^(-3). The...
Text Solution
|
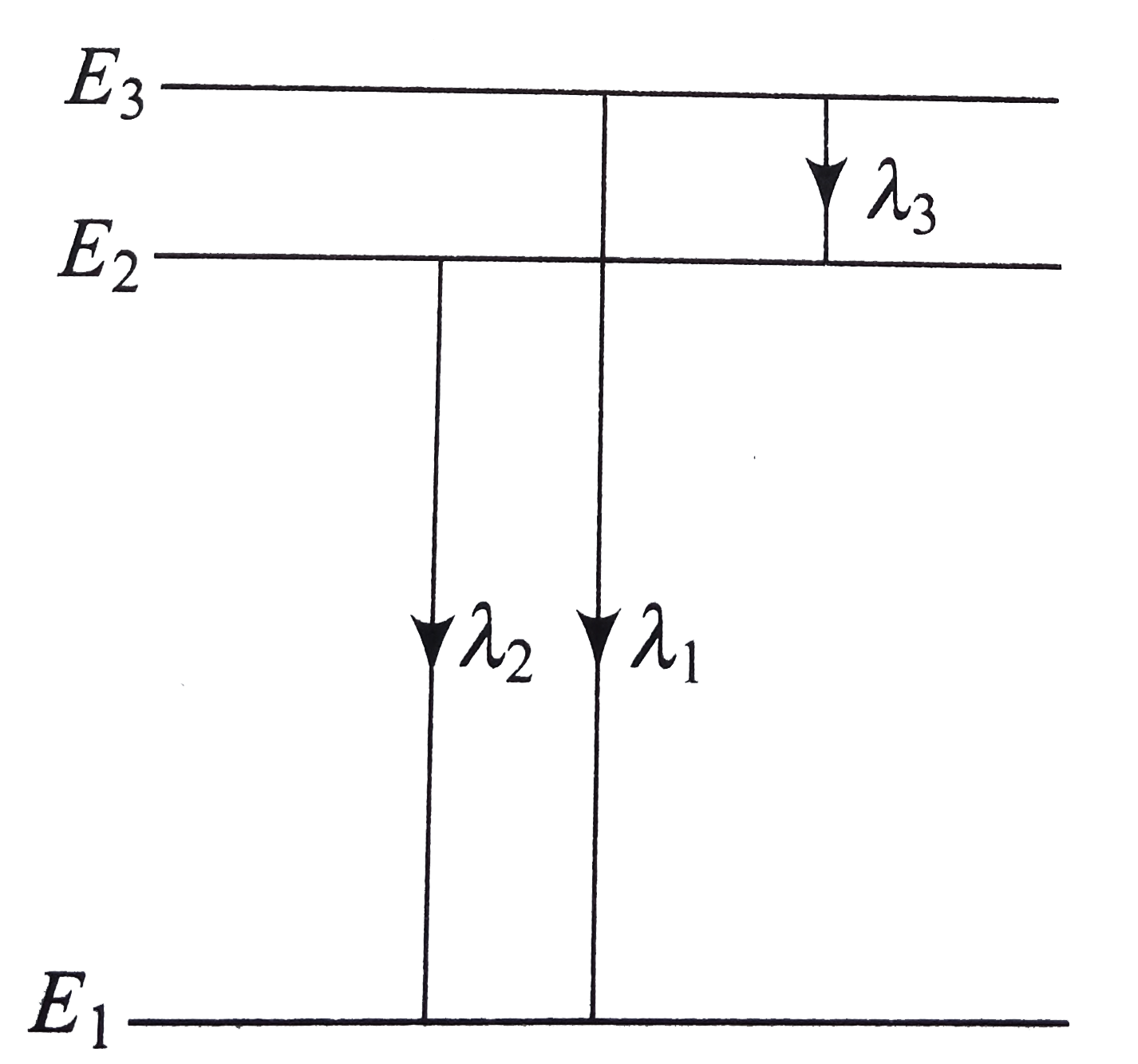
- Three energy levels of an atom are shown in figure . The wavelength co...
Text Solution
|
- The ratio of the speed of the electron in the first Bohr orbit of hydr...
Text Solution
|
- Suppose two deuterons must get as close as 10^(-14) m in order for the...
Text Solution
|
- An electron in H atom makes a transition from n = 3 to n = 1. The reco...
Text Solution
|
- A hydrogen-like atom emits radiation of frequency 2.7 xx 10^(15) Hz wh...
Text Solution
|
- An electron in a hydrogen atom makes a trsnsition n(1)rarrn(2) where n...
Text Solution
|
- An electron revolving in an orbit of radius 0.5 Å in a hydrogen atom e...
Text Solution
|
- The total energy of an electron in the ground state of hydrogen atom i...
Text Solution
|
- A doubly ionized lithium atom is hydrogen like atom with atomic . numb...
Text Solution
|
- In Bohr modal of hydrogen atom, the force on the electron depends on t...
Text Solution
|
- The minimum energy to ionize an atom is the energy required to
Text Solution
|
- If electron with principal quantum number n gt 4 were not allowed in n...
Text Solution
|
- The orbital velocity of electron in the ground state is v. If the elec...
Text Solution
|
- If potential energy between a proton and an electron is given by | U |...
Text Solution
|
- In H-atom , a transition takes place from n=3 to n=2 orbit. Calculate ...
Text Solution
|
- An alpha particle of energy 5 MeV is scattered through 180^(@) by a fi...
Text Solution
|
- How many time does the electron go round the first bohr orbit of hydro...
Text Solution
|
- The radius of hydrogen atom in its ground state is 5.3xx10^(-11)m. Aft...
Text Solution
|