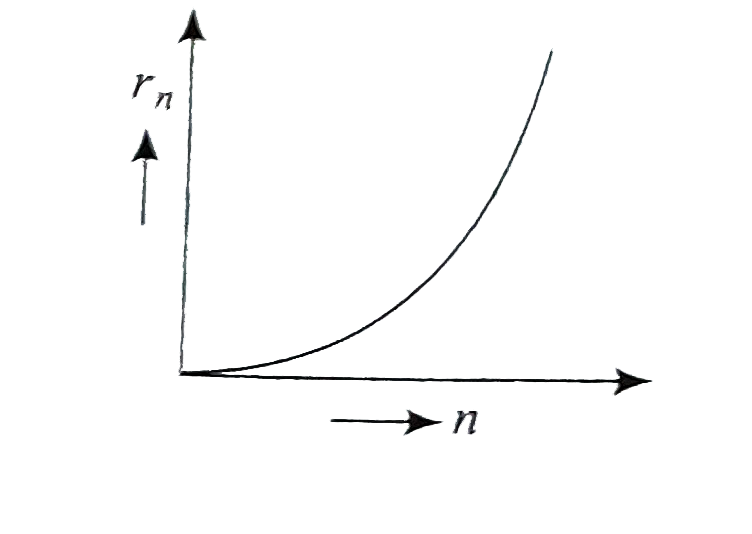
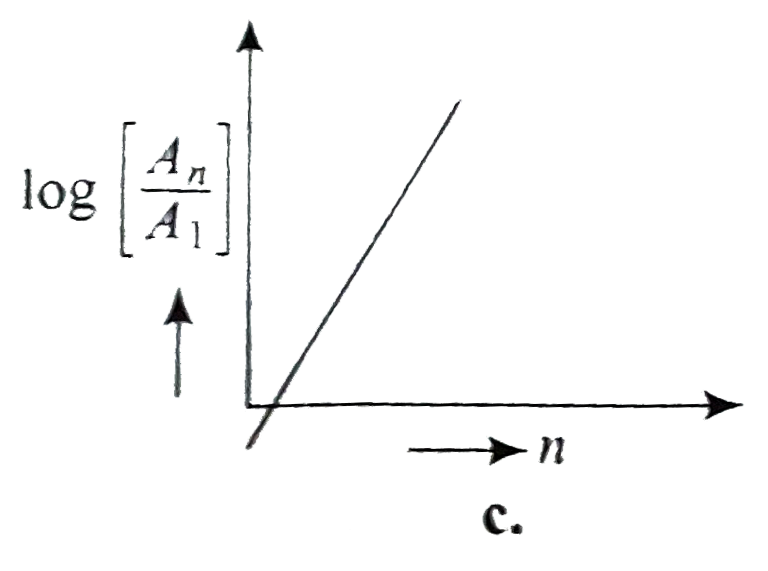
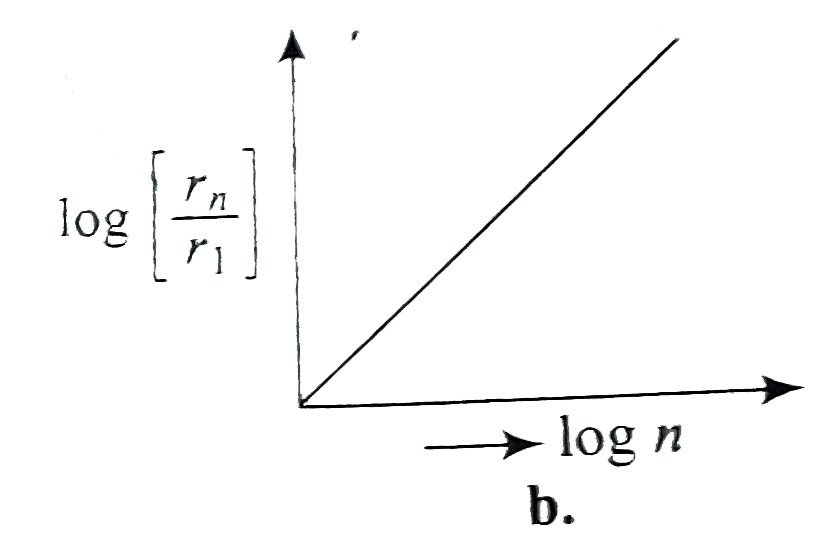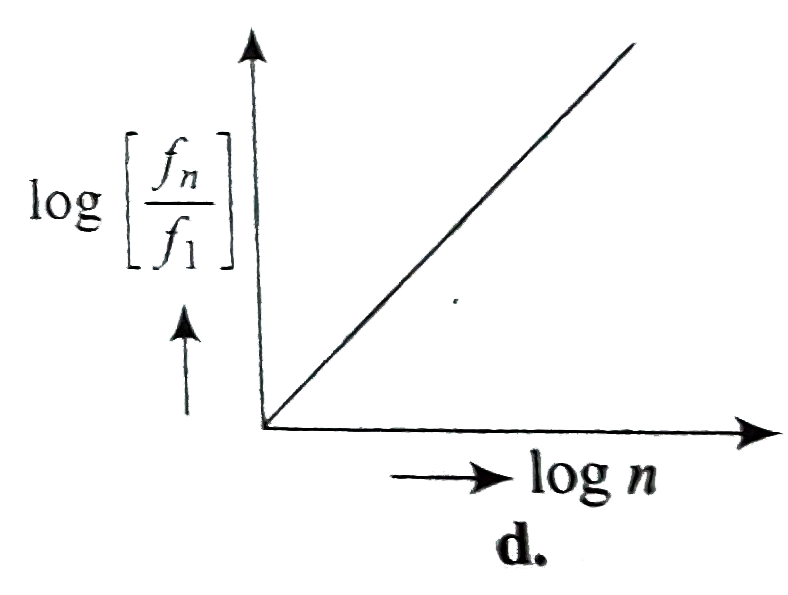A
B
C
D
Text Solution
AI Generated Solution
The correct Answer is:
Topper's Solved these Questions
ATOMIC PHYSICS
CENGAGE PHYSICS ENGLISH|Exercise Multiple Correct|13 VideosATOMIC PHYSICS
CENGAGE PHYSICS ENGLISH|Exercise Linked Comprehension|62 VideosATOMIC PHYSICS
CENGAGE PHYSICS ENGLISH|Exercise Subject|17 VideosALTERNATING CURRENT
CENGAGE PHYSICS ENGLISH|Exercise Single Correct|10 VideosCAPACITOR AND CAPACITANCE
CENGAGE PHYSICS ENGLISH|Exercise Integer|5 Videos
Similar Questions
Explore conceptually related problems
CENGAGE PHYSICS ENGLISH-ATOMIC PHYSICS-Single Correct
- Which of the following products, in a hydrogen atom , are independent ...
Text Solution
|
- According to Bohr's theory of hydrogen atom , for the electron in the...
Text Solution
|
- When a hydrogen atom is excited from ground state to first excited st...
Text Solution
|
- In an X-ray tube, the voltage applied is 20 kV. The energy required to...
Text Solution
|
- Supose the potential energy between electron and proton at a distance ...
Text Solution
|
- Let An be the area enclosed by the nth orbit in a hydrogen atom. Th...
Text Solution
|
- Mark out the correct statement(s).
Text Solution
|
- A hydrogen atom having kinetic energy E collides with a stationary hyd...
Text Solution
|
- In Bohr's model of hydrogen atom ,
Text Solution
|
- Which of the following are in the ascending order of wavelength?
Text Solution
|
- Continuous spectrum is produced by
Text Solution
|
- A gas of monoatomic hydrogen is bombarded with a stream of electrons t...
Text Solution
|
- If the potential energy of the electron in the first allowed orbit in ...
Text Solution
|
- According to Bohr's theory of hydrogen atom , for the electron in the...
Text Solution
|
- Whenever a hydrogen atom emitsa photon in the Balmer series .
Text Solution
|
- Let lambda(alpha), lambda(beta),and lambda'(alpha) denote the wavelen...
Text Solution
|
- Which of the following statements are correct for an X-ray tube?
Text Solution
|
- Suppose frequency of emitted photon is f(0) when the electron of a sta...
Text Solution
|
- Which of the following statements about hydrogen spectrum are correct?
Text Solution
|
- If, in a hydrogen atom, radius of nth Bohr orbit is r(n) frequency of ...
Text Solution
|