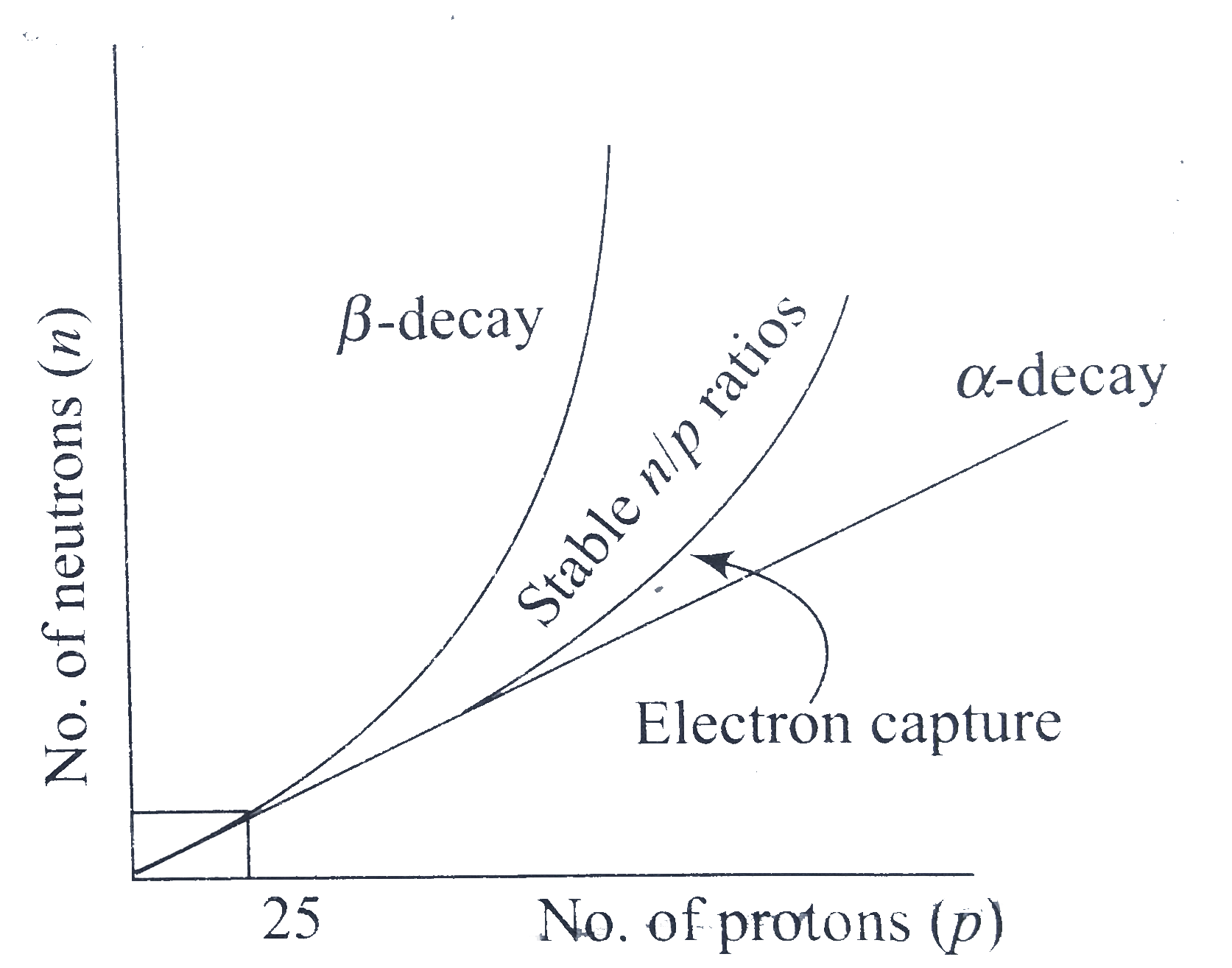
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
NUCLEAR PHYSICS
CENGAGE PHYSICS ENGLISH|Exercise Integer|6 VideosNUCLEAR PHYSICS
CENGAGE PHYSICS ENGLISH|Exercise Fill In The Blanks|5 VideosNUCLEAR PHYSICS
CENGAGE PHYSICS ENGLISH|Exercise Single Correct Option|182 VideosMISCELLANEOUS VOLUME 5
CENGAGE PHYSICS ENGLISH|Exercise Integer|12 VideosPHOTOELECTRIC EFFECT
CENGAGE PHYSICS ENGLISH|Exercise Integer Type|4 Videos
Similar Questions
Explore conceptually related problems
CENGAGE PHYSICS ENGLISH-NUCLEAR PHYSICS-Linked Comprehension
- A radioactive with decay constant lambda is being produced in a nuclea...
Text Solution
|
- Various rules of thumb have seen proposed by the scientific community ...
Text Solution
|
- Various rules of thumb have seen proposed by the scientific community ...
Text Solution
|
- Various rules of thumb have seen proposed by the scientific community ...
Text Solution
|
- The radionuclide .^(56)Mn is being produced in a cyclontron at a const...
Text Solution
|
- The radionuclide .^(56)Mn is being produced in a cyclontron at a const...
Text Solution
|
- The radionuclide .^(56)Mn is being produced in a cyclontron at a const...
Text Solution
|
- Many unstable nuclie can decay spontaneously to a nucleus of lower mas...
Text Solution
|
- Many unstable nuclie can decay spontaneously to a nucleus of lower mas...
Text Solution
|
- Many unstable nuclie can decay spontaneously to a nucleus of lower mas...
Text Solution
|
- All nuclei consist of two types of particles- protaon and neutrons. Nu...
Text Solution
|
- All nuclei consist of two types of particles- protaon and neutrons. Nu...
Text Solution
|
- All nuclei consist of two types of particles- protaon and neutrons. Nu...
Text Solution
|
- The compound unstabel nucleus .(92)^(236)U often decays in accordance ...
Text Solution
|
- The compound unstabel nucleus .(92)^(236)U often decays in accordance ...
Text Solution
|
- The compound unstabel nucleus .(92)^(236)U often decays in accordance ...
Text Solution
|
- The compound unstabel nucleus .(92)^(236)U often decays in accordance ...
Text Solution
|
- A beam of alpha paricles is incident on a target of lead. A particular...
Text Solution
|
- A beam of alpha paricles is incident on a target of lead. A particular...
Text Solution
|
- A beam of alpha paricles is incident on a target of lead. A particular...
Text Solution
|