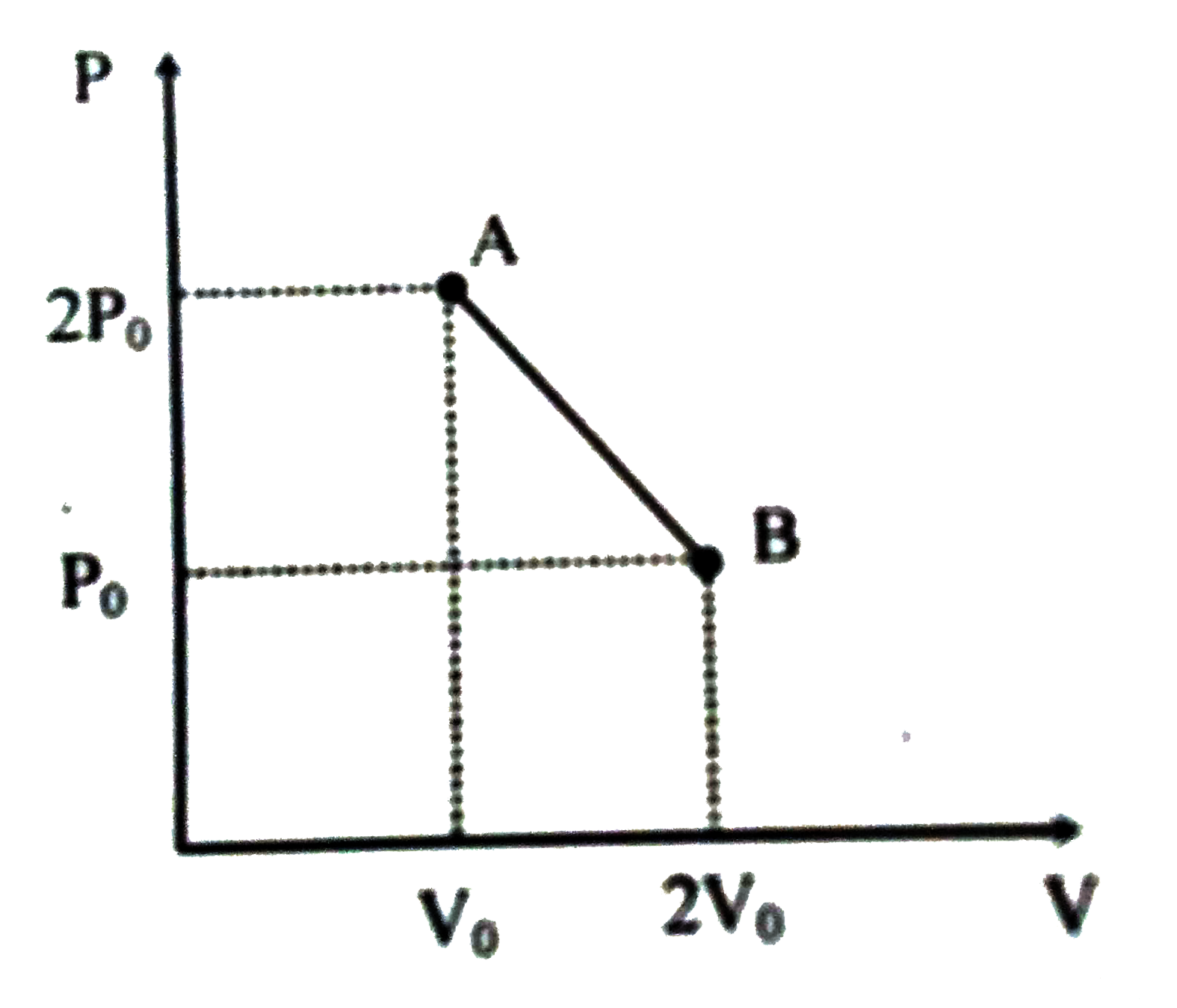
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
KINETIC THEORY OF GASES AND FIRST LAW OF THERMODYNAMICS
CENGAGE PHYSICS ENGLISH|Exercise Multiple Corrects|29 VideosKINETIC THEORY OF GASES AND FIRST LAW OF THERMODYNAMICS
CENGAGE PHYSICS ENGLISH|Exercise Assertion-Reasoning|6 VideosKINETIC THEORY OF GASES AND FIRST LAW OF THERMODYNAMICS
CENGAGE PHYSICS ENGLISH|Exercise Subjective|22 VideosKINETIC THEORY OF GASES
CENGAGE PHYSICS ENGLISH|Exercise Compression|2 VideosLINEAR AND ANGULAR SIMPLE HARMONIC MOTION
CENGAGE PHYSICS ENGLISH|Exercise Single correct anwer type|14 Videos
Similar Questions
Explore conceptually related problems
CENGAGE PHYSICS ENGLISH-KINETIC THEORY OF GASES AND FIRST LAW OF THERMODYNAMICS-Single Correct
- Three samples A, B and C of the same gas (gamma = 1.5) have equal volu...
Text Solution
|
- p-V diagram of an ideal gas is as shown in figure. Work done by the ga...
Text Solution
|
- ‘n’ moles of an ideal gas undergoes a process A to B as shown in the f...
Text Solution
|
- The relation between internal energy U, pressure P and volume V of a g...
Text Solution
|
- One mole of an ideal gas at temperature T(1) expands slowly according ...
Text Solution
|
- Two moles of an ideal gas at temperature T(0) = 300 K was cooled isoch...
Text Solution
|
- An ideal gas is taken around the cycle ABCA shown in P - V diagram. Th...
Text Solution
|
- Heat energy absorbed by a system is going through a cyclic process sho...
Text Solution
|
- A diatomic ideal gas is heated at constant volume until the pressure ...
Text Solution
|
- One mole of an ideal gas is taken from state A to state B by three dif...
Text Solution
|
- One mole of an ideal gas at pressure P(0) and temperature T(0) is expa...
Text Solution
|
- P-T diagram is shown below. Then choose the corresponding V-T diagram
Text Solution
|
- P-V curve of a diatomic gas is shown in the Fig. Find the total heat g...
Text Solution
|
- A gas expands with temperature according to the relation V = kT^(2//3)...
Text Solution
|
- A gas is expanded from volume V(0) to 2V(0) under three different proc...
Text Solution
|
- Logarithms of readings of pressure and volume for an ideal gas were pl...
Text Solution
|
- Two moles of an ideal mono-atomic gas undergo a cyclic process as show...
Text Solution
|
- ‘n’ moles of an ideal gas undergoes a process A to B as shown in the f...
Text Solution
|
- A vessel contains a mixture consisting of m1=7 g of nitrogen (M1=28) a...
Text Solution
|
- The mass of a gas molecule can be computed form the specific heat at c...
Text Solution
|