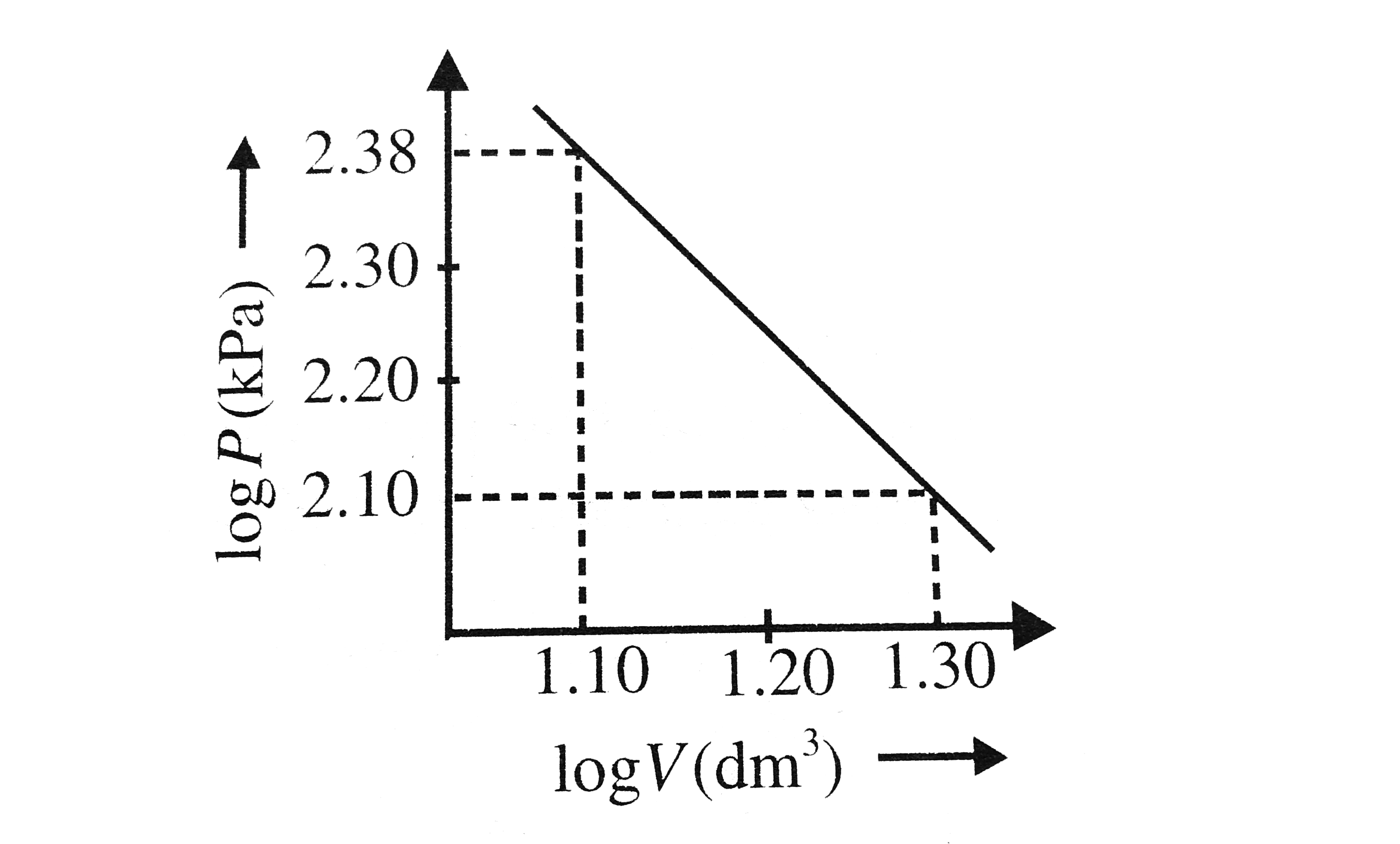A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
KINETIC THEORY OF GASES AND FIRST LAW OF THERMODYNAMICS
CENGAGE PHYSICS ENGLISH|Exercise Multiple Corrects|29 VideosKINETIC THEORY OF GASES AND FIRST LAW OF THERMODYNAMICS
CENGAGE PHYSICS ENGLISH|Exercise Assertion-Reasoning|6 VideosKINETIC THEORY OF GASES AND FIRST LAW OF THERMODYNAMICS
CENGAGE PHYSICS ENGLISH|Exercise Subjective|22 VideosKINETIC THEORY OF GASES
CENGAGE PHYSICS ENGLISH|Exercise Compression|2 VideosLINEAR AND ANGULAR SIMPLE HARMONIC MOTION
CENGAGE PHYSICS ENGLISH|Exercise Single correct anwer type|14 Videos
Similar Questions
Explore conceptually related problems
CENGAGE PHYSICS ENGLISH-KINETIC THEORY OF GASES AND FIRST LAW OF THERMODYNAMICS-Single Correct
- A gas expands with temperature according to the relation V = kT^(2//3)...
Text Solution
|
- A gas is expanded from volume V(0) to 2V(0) under three different proc...
Text Solution
|
- Logarithms of readings of pressure and volume for an ideal gas were pl...
Text Solution
|
- Two moles of an ideal mono-atomic gas undergo a cyclic process as show...
Text Solution
|
- ‘n’ moles of an ideal gas undergoes a process A to B as shown in the f...
Text Solution
|
- A vessel contains a mixture consisting of m1=7 g of nitrogen (M1=28) a...
Text Solution
|
- The mass of a gas molecule can be computed form the specific heat at c...
Text Solution
|
- A certain balloon maintains an internal gas pressure of P(0) = 100 k P...
Text Solution
|
- A spherical balloon contains air at temperature T(0) and pressure P(0)...
Text Solution
|
- Find work done by the gas in the process shown in figure.
Text Solution
|
- Two different ideal diatomic gases A and B are initially in the same s...
Text Solution
|
- Three moles of an ideal monoatomic gas performs a cyclic process as sh...
Text Solution
|
- For process 1, Delta U is positive, for process 2, Delta U is zero and...
Text Solution
|
- In the diagram as shown, find parameters representing x and y axes and...
Text Solution
|
- The ratio of heat absorbed and work done by the gas in the process, as...
Text Solution
|
- In previous, Find the value of gamma = C(P) // C(v) :
Text Solution
|
- In an adiabatic process, R = (2)/(3) C(v). The pressure of the gas wil...
Text Solution
|
- The ratio of pressure of the same gas in two containers is (n(1) T(1))...
Text Solution
|
- In an isobaric process, Delta Q = (K gamma)/(gamma - 1) where gamma = ...
Text Solution
|
- On an isothermal process, there are two POINTs A and B at which pressu...
Text Solution
|