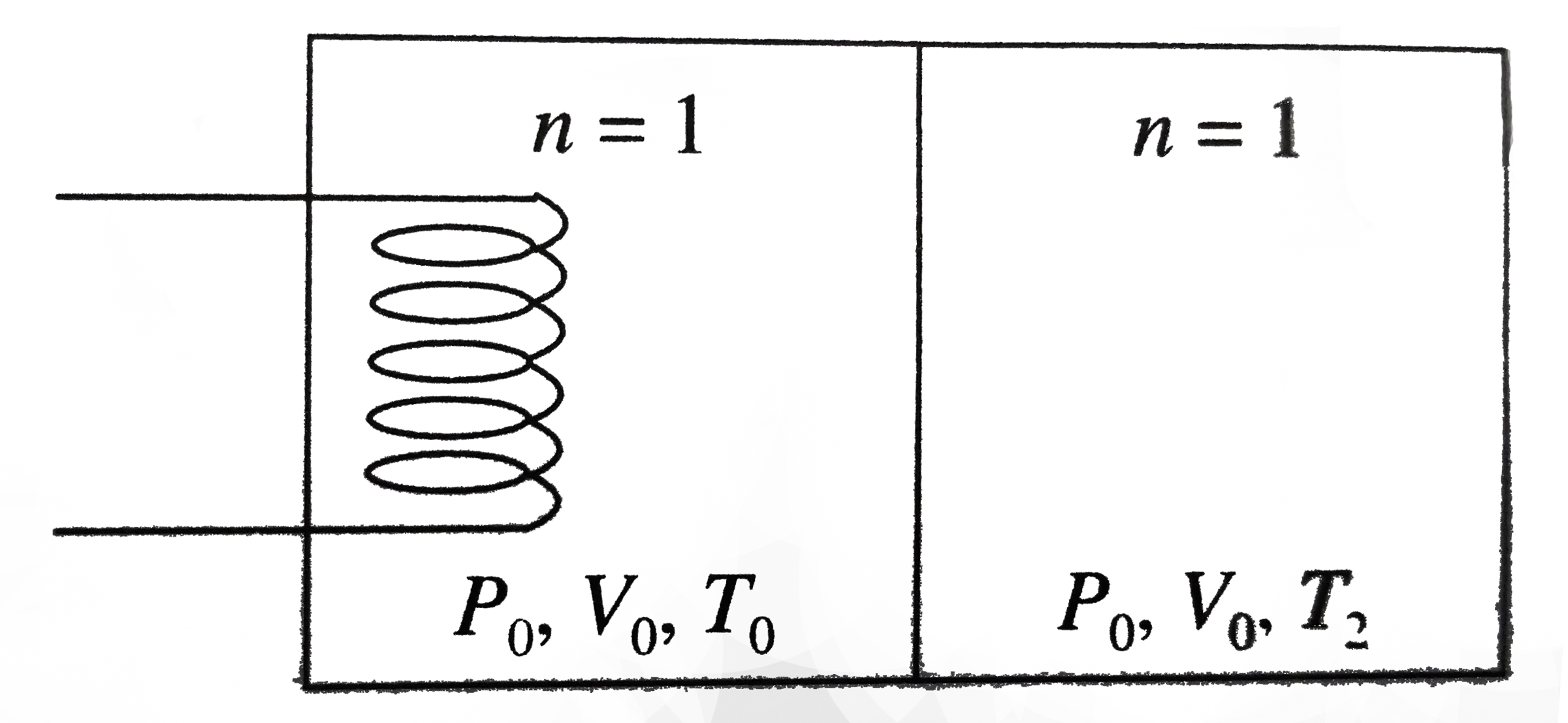
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
KINETIC THEORY OF GASES AND FIRST LAW OF THERMODYNAMICS
CENGAGE PHYSICS ENGLISH|Exercise Interger|11 VideosKINETIC THEORY OF GASES AND FIRST LAW OF THERMODYNAMICS
CENGAGE PHYSICS ENGLISH|Exercise Assertion-Reasoning|6 VideosKINETIC THEORY OF GASES
CENGAGE PHYSICS ENGLISH|Exercise Compression|2 VideosLINEAR AND ANGULAR SIMPLE HARMONIC MOTION
CENGAGE PHYSICS ENGLISH|Exercise Single correct anwer type|14 Videos
Similar Questions
Explore conceptually related problems
CENGAGE PHYSICS ENGLISH-KINETIC THEORY OF GASES AND FIRST LAW OF THERMODYNAMICS-Comprehension
- A monatomic gas undergoes a cycle consisting of two isothermals and tw...
Text Solution
|
- A monatomic gas undergoes a cycle consisting of two isothermals and tw...
Text Solution
|
- A monatomic gas undergoes a cycle consisting of two isothermals and tw...
Text Solution
|
- A monoatomic gas undergoes a cycle consisting of two isothermals and t...
Text Solution
|
- Figure shows an insulated cylinder of volume V containing monatomic g...
Text Solution
|
- Figure shows an insulated cylinder of volume V containing monatomic g...
Text Solution
|
- Figure shows an insulated cylinder of volume V containing monatomic g...
Text Solution
|
- Figure shows an insulated cylinder of volume V containing monatomic g...
Text Solution
|
- Figure shows an insulated cylinder of volume V containing monatomic g...
Text Solution
|
- The rectangular box shown in Fig has partition which can slide without...
Text Solution
|
- The insulated box shown in figure has an insulated partition which can...
Text Solution
|
- A container of volume 4V(0) made of a perfectly non- conducting materi...
Text Solution
|
- A container of volume 4V(0) made of a perfectly non- conducting materi...
Text Solution
|
- A container of volume 4V(0) made of a perfectly non- conducting materi...
Text Solution
|
- An ideal gas at NTP is enclosed in an adiabatic vertical cylinder havi...
Text Solution
|
- An ideal gas at NTP is enclosed in an adiabatic vertical cylinder havi...
Text Solution
|
- An ideal gas at NTP is enclosed in an adiabatic vertical cylinder havi...
Text Solution
|
- Piston cylinder device initially contains 0.5m^(3) of nitrogen gas at ...
Text Solution
|
- Piston cylinder device initially contains 0.5m^(3) of nitrogen gas at ...
Text Solution
|
- Piston cylinder device initially contains 0.5m^(3) of nitrogen gas at ...
Text Solution
|