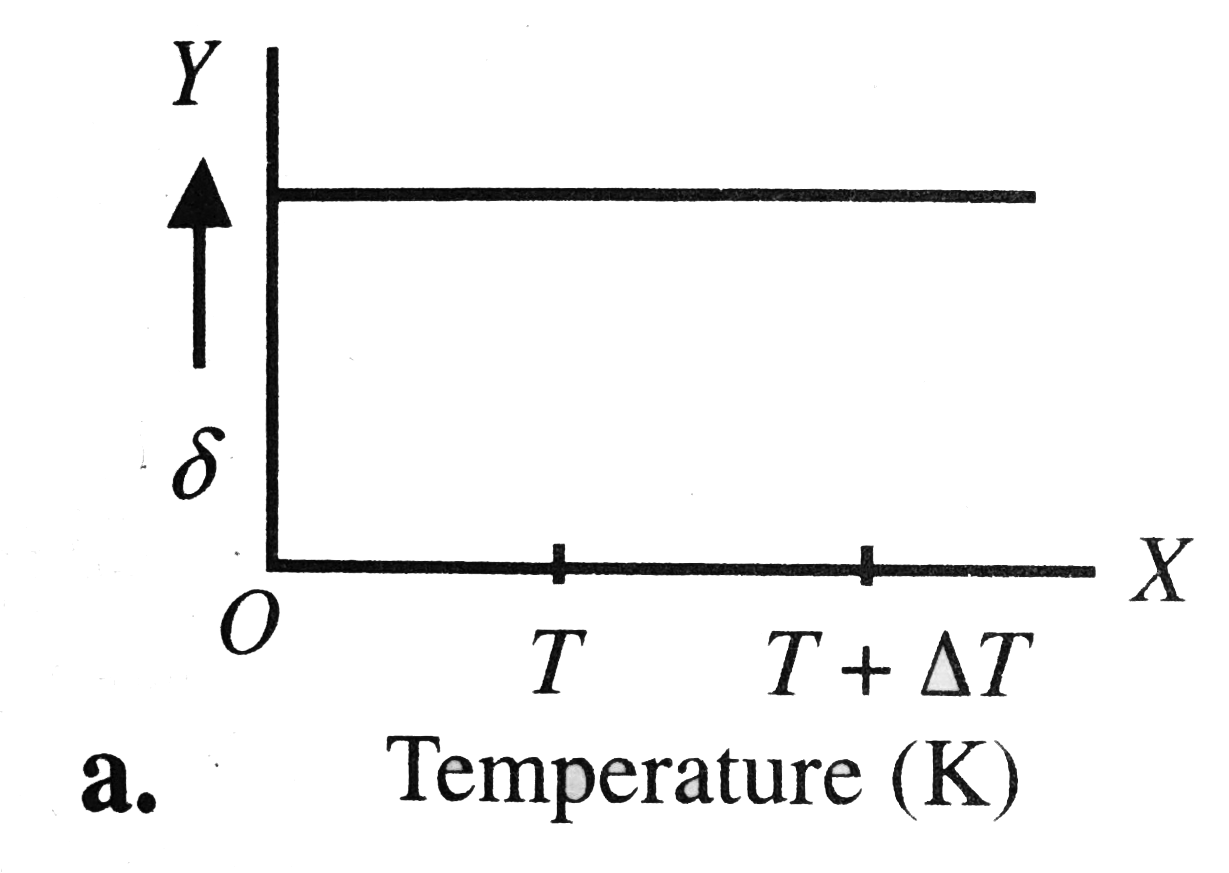
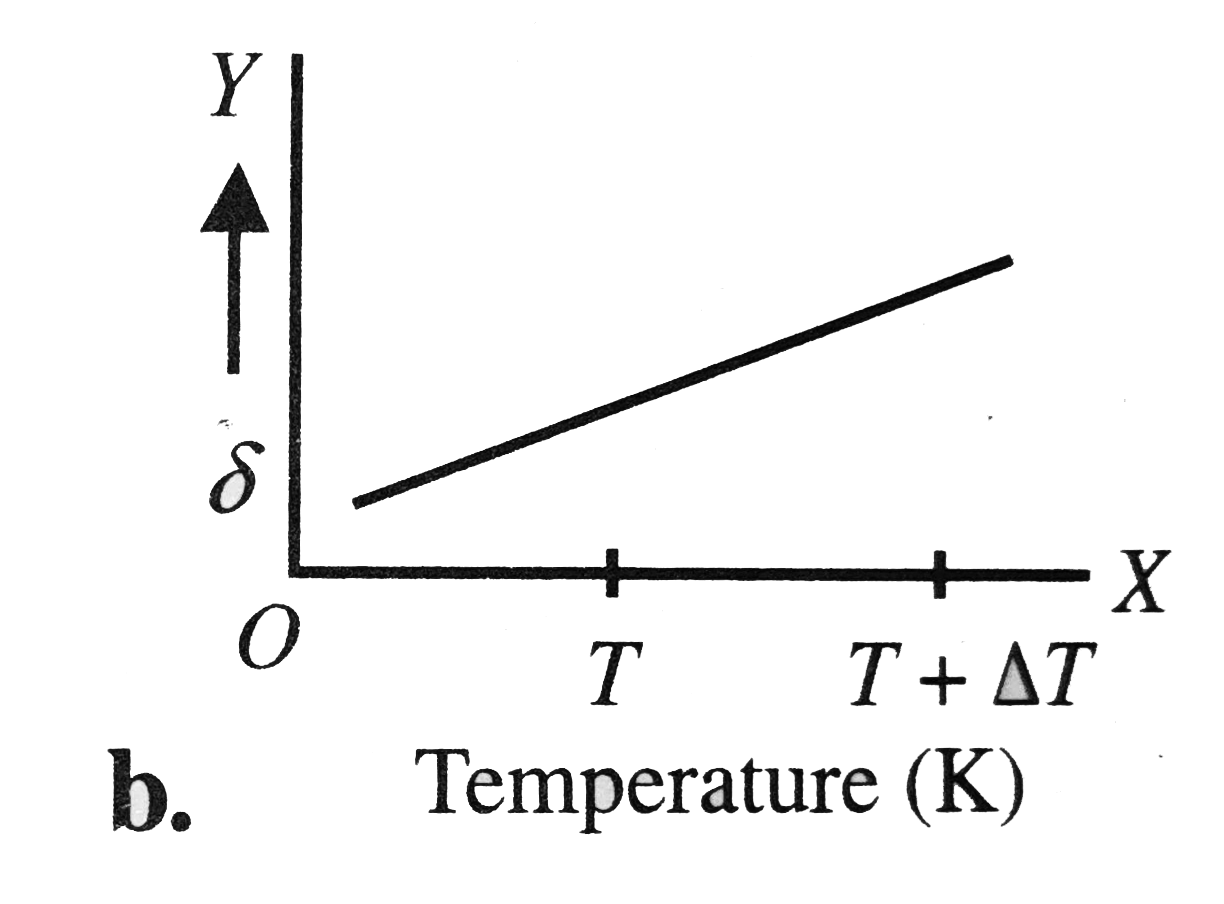
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
CENGAGE PHYSICS ENGLISH-ARCHIVES 1 VOLUME 6-Single Correct
- A monatomic ideal gas, initially at temperature T(1) is enclosed in a ...
Text Solution
|
- A block of ice at -8^(@)C is slowly heated and converted to steam ...
Text Solution
|
- An ideal gas is initially at temperature T and volume V. ITS volume is...
Text Solution
|
- Starting from the same initial conditions, an ideal gas expands from v...
Text Solution
|
- The plots of intensity versus wavelength fro three black bodies at tem...
Text Solution
|
- Three rods made of the same material and having the same cross-section...
Text Solution
|
- In a given process on an ideal gas, dW = 0 and dQ lt 0. Then for the g...
Text Solution
|
- P-V plots for two gases during adiabatic processes are shown in Fig. 1...
Text Solution
|
- An ideal gas is taken through cycle A to B to C-A, as shown in Fig. IF...
Text Solution
|
- Which of the following graphs correctly represents the variation of be...
Text Solution
|
- An ideal black body at room temperature is thrown into a furnance.It i...
Text Solution
|
- The graph, shown in the adjacement diagram, represents the variation o...
Text Solution
|
- Two rods, one of aluminium and the other made of steel, having initial...
Text Solution
|
- The PT diagram for an ideal gas is shown in the figure, where AC is an...
Text Solution
|
- 2kg of ice at -20^@C is mixed with 5kg of water at 20^@C in an insulat...
Text Solution
|
- Three discs, A, B and C having radii 2m, 4m and6m respectively are coa...
Text Solution
|
- Liquefied oxygen at 1 atmosphere is heated from 50 K to 300 K by suppl...
Text Solution
|
- Two identical rods are connected between two containers. One of them i...
Text Solution
|
- An ideal gas is initially at P1,V1 is expands to P2,V2 isothermally an...
Text Solution
|
- Variation of radiant energy emitted by sun, filament of tungsten lamp ...
Text Solution
|