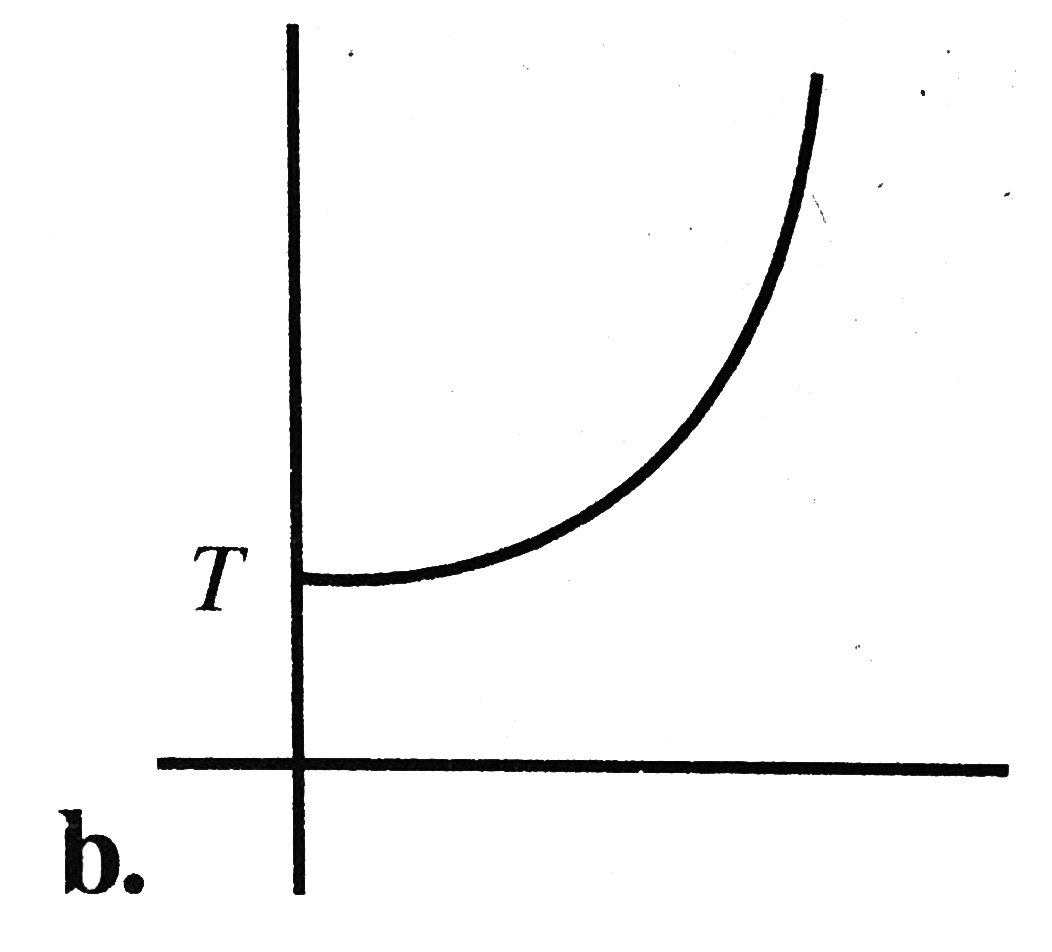
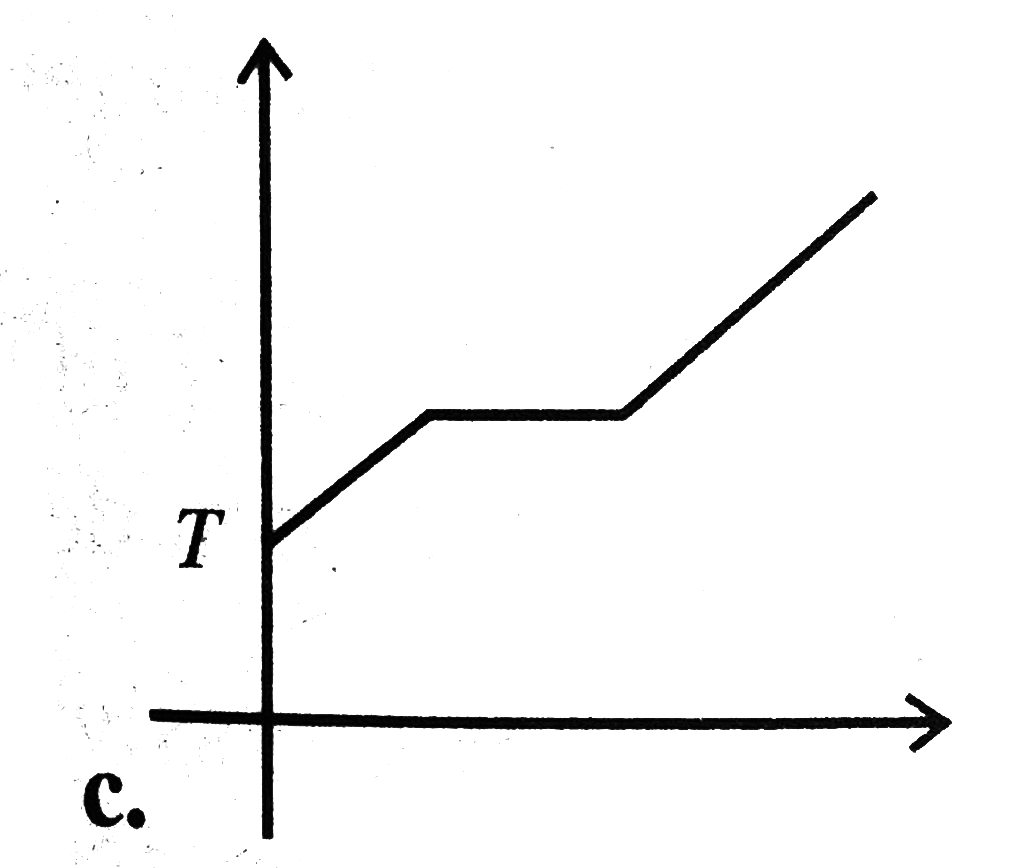
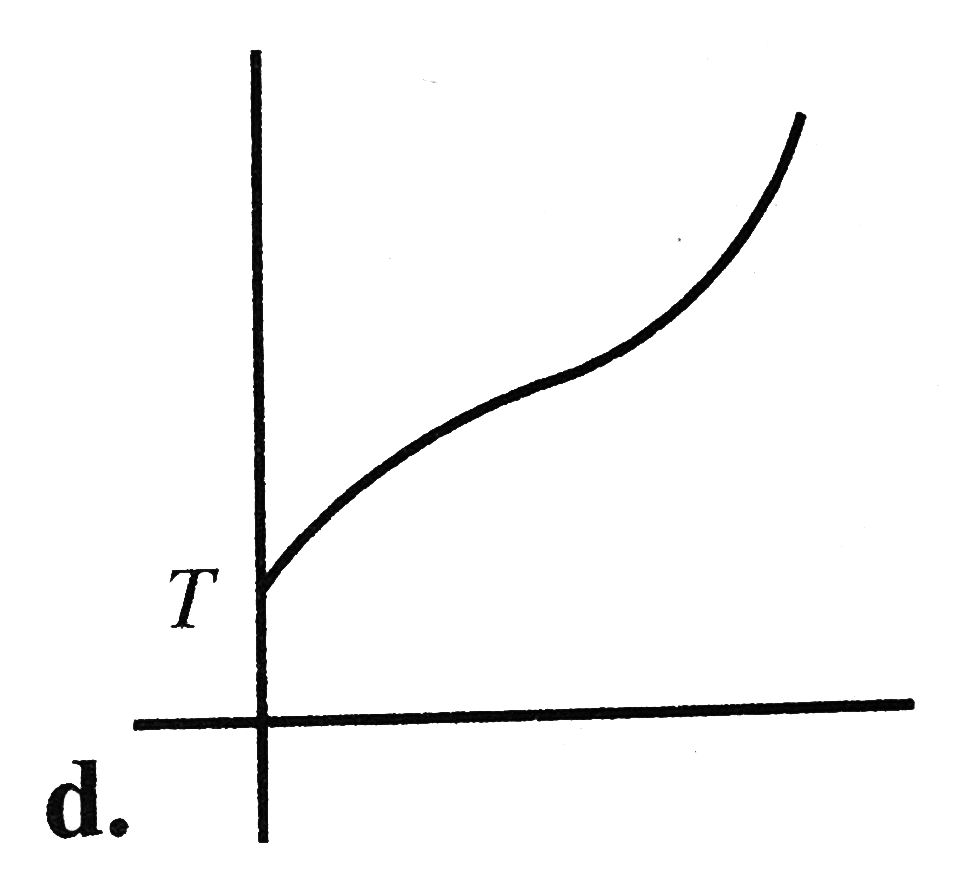
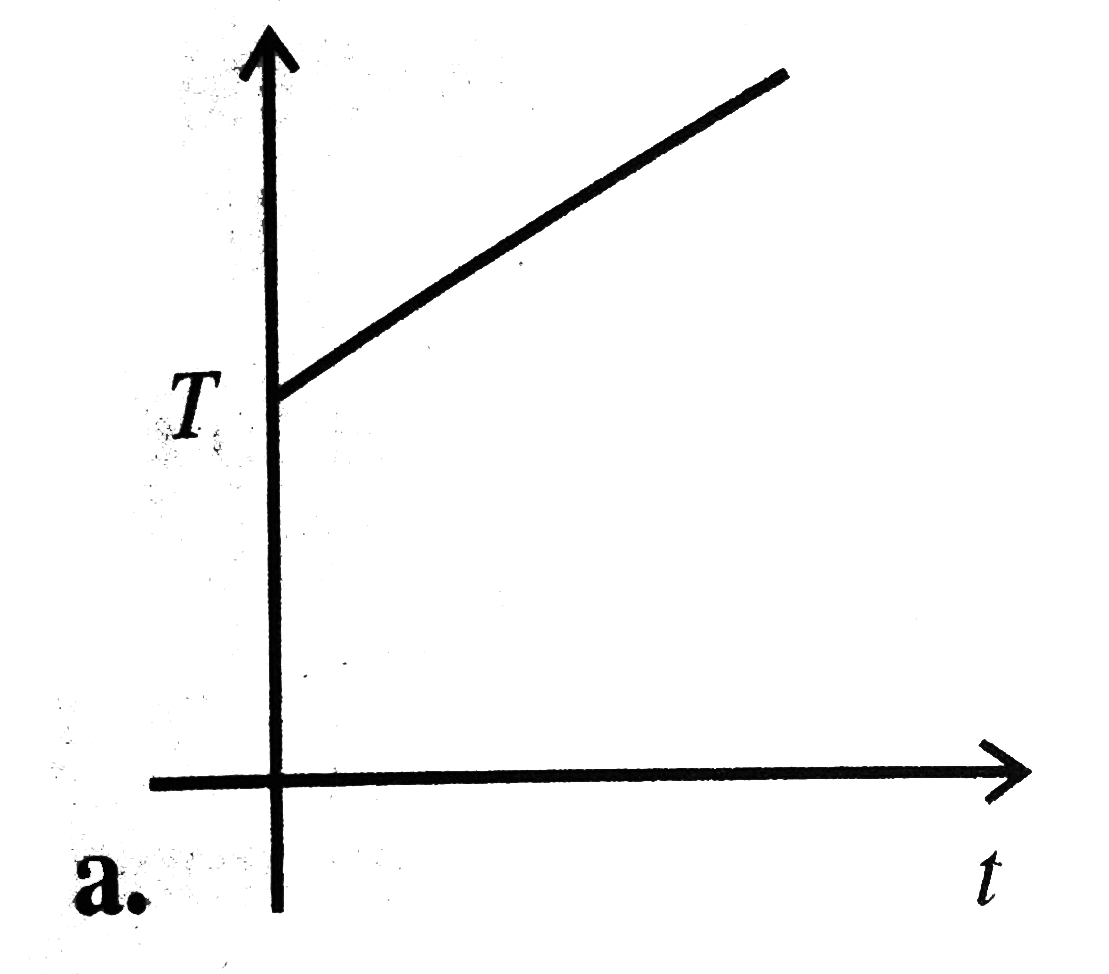A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
CENGAGE PHYSICS ENGLISH-ARCHIVES 1 VOLUME 6-Single Correct
- Two rods, one of aluminium and the other made of steel, having initial...
Text Solution
|
- The PT diagram for an ideal gas is shown in the figure, where AC is an...
Text Solution
|
- 2kg of ice at -20^@C is mixed with 5kg of water at 20^@C in an insulat...
Text Solution
|
- Three discs, A, B and C having radii 2m, 4m and6m respectively are coa...
Text Solution
|
- Liquefied oxygen at 1 atmosphere is heated from 50 K to 300 K by suppl...
Text Solution
|
- Two identical rods are connected between two containers. One of them i...
Text Solution
|
- An ideal gas is initially at P1,V1 is expands to P2,V2 isothermally an...
Text Solution
|
- Variation of radiant energy emitted by sun, filament of tungsten lamp ...
Text Solution
|
- In which of the following process convection of does not take plac...
Text Solution
|
- A spherical body of area A and emissivity e=0.6 is kept inside a perfe...
Text Solution
|
- Calorie is defined as the amount of heat required to raise temperature...
Text Solution
|
- A kettle with 2 litre water at 27^@C is heated by operating coil heate...
Text Solution
|
- An ideal gas is expanding such that PT^2=constant. The coefficient of ...
Text Solution
|
- A real gas behaves like an ideal gas if its
Text Solution
|
- 5.6 liter of helium gas at STP is adiabatically compressed to 0.7 lite...
Text Solution
|
- Three very large plates of same area are kept parallel and close to ea...
Text Solution
|
- A mixture of 2 moles of helium gas ( (atomic mass)=4a.m.u ) and 1 mole...
Text Solution
|
- Two moles of ideal helium gas are in a rubber balloon at 30^@C. The ba...
Text Solution
|
- Two rectangular blocks, having identical dimensions, an be arranged ei...
Text Solution
|
- Two non-reactive monoatomic ideal gases have their atomic masses in th...
Text Solution
|