A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
SOME BASIC CONCEPTS AND MOLE CONCEPT
CENGAGE CHEMISTRY ENGLISH|Exercise Exercises Assertion-Reasoning|10 VideosSOME BASIC CONCEPTS AND MOLE CONCEPT
CENGAGE CHEMISTRY ENGLISH|Exercise Exercises Integers|13 VideosSOME BASIC CONCEPTS AND MOLE CONCEPT
CENGAGE CHEMISTRY ENGLISH|Exercise Exercises Multiple Correct (Mole Concept In Solution)|15 VideosS-BLOCK GROUP 2 - ALKALINE EARTH METALS
CENGAGE CHEMISTRY ENGLISH|Exercise Ex 5.1 Objective|2 VideosSTATES OF MATTER
CENGAGE CHEMISTRY ENGLISH|Exercise Exercises (Ture False)|25 Videos
Similar Questions
Explore conceptually related problems
CENGAGE CHEMISTRY ENGLISH-SOME BASIC CONCEPTS AND MOLE CONCEPT-Exercises Single Correct
- The weight of 1 xx 10^22 molecules of CuSO4. 5H2O is:
Text Solution
|
- How many moles of O(2) will be liberated by one mole of CrO(5) is the ...
Text Solution
|
- BrO(3)^(ɵ) + 5Br^(ɵ) rarr Br(2) + 3 H(2) O IF 50 mL 0.1 M BrO(3)^(ɵ)...
Text Solution
|
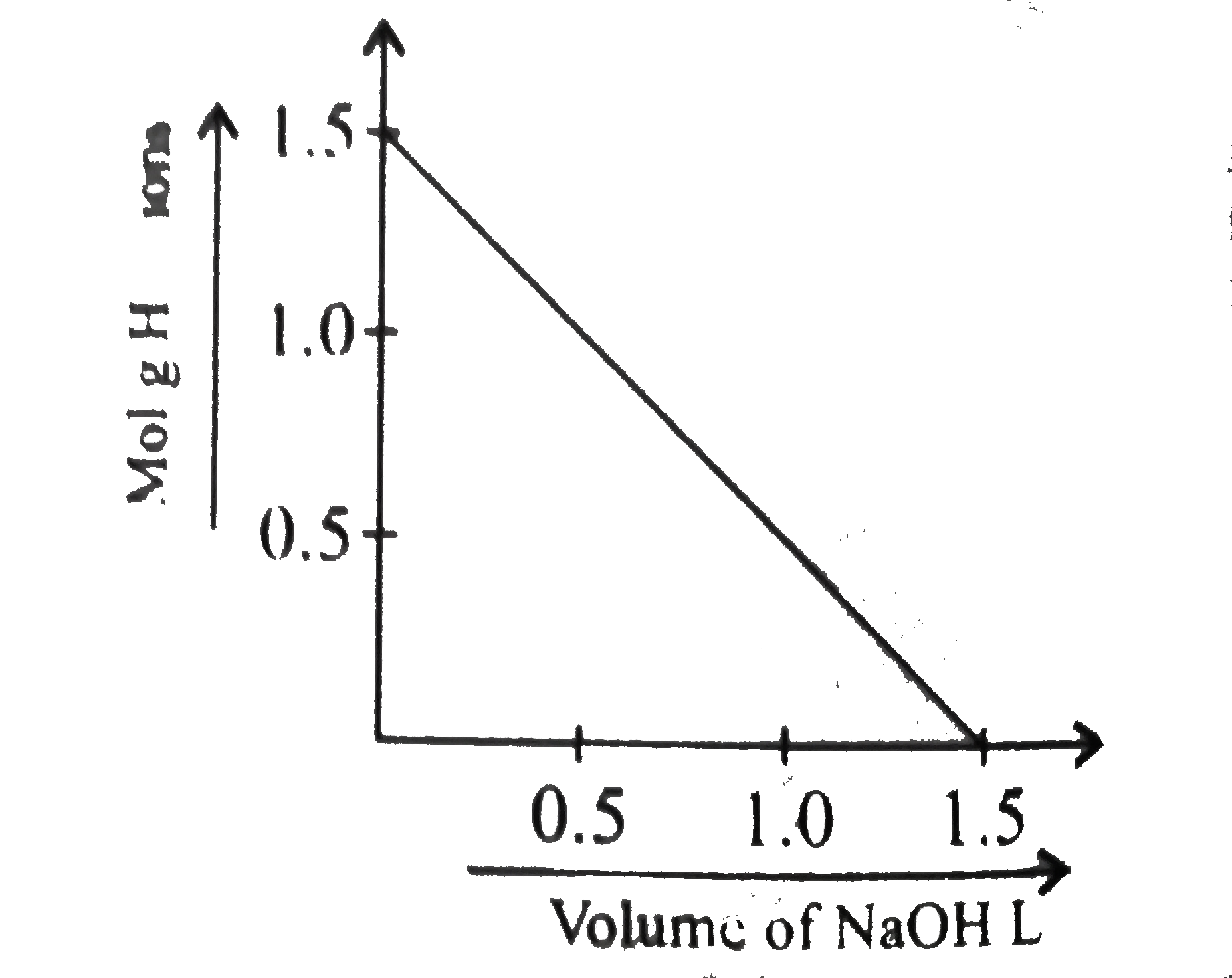
- To 1 L of 1.0 M impure H(2)SO(4) sample, 1.0 M NaOH solution was added...
Text Solution
|
- The expression relating mole fraction of solute (chi(2)) and molarity ...
Text Solution
|
- At 100°C and 1 atm, if the density of liquid water is 1.0 g cm^(-3) an...
Text Solution
|
- Consider the ionisation of H(2) SO(4) as follow" H(2) SO(4) + 2H(2)P...
Text Solution
|
- Calculate the number of oxygen atoms requried to combine with 7.0 g of...
Text Solution
|
- 36.5% HCl has density has density equal to 1.20 g mL^(-1). The molarit...
Text Solution
|
- 10 mL of 1 M BaCl(2) solution & 5 mL 0.5 M K(2)SO(4) are mixed togethe...
Text Solution
|
- Mole fraction of a solute in an aqueous solution is 0.2. The molality ...
Text Solution
|
- An excess of NaOH was added to 100 mL of a FeCl(3) solution which give...
Text Solution
|
- Two samples of HCl of 1.0 M and 0.25 M are mixed. Find volumes of thes...
Text Solution
|
- If 100 mL of H(2) SO(4) and 100 mL of H(2)O are mixed, the mass percen...
Text Solution
|
- 12.5 mL of a solution containing 6.0 g of a dibasic acid in 1 L was fo...
Text Solution
|
- One litre of a sample of hard water contains 5.55 mg of CaCl(2) and 4....
Text Solution
|
- 10 mL of 0.2 N HCl and 30 mL of 0.1 N HCl to gether exaclty neutralise...
Text Solution
|
- A metal oxide has the formul Z(2)O(3). It can be reduced by hydrogen t...
Text Solution
|
- The reaction between yttrium metal and dilute HCl produces H(2) (g) an...
Text Solution
|
- What volume of H(2) at 273 K and 1 atm will be consumed in obtaining 2...
Text Solution
|