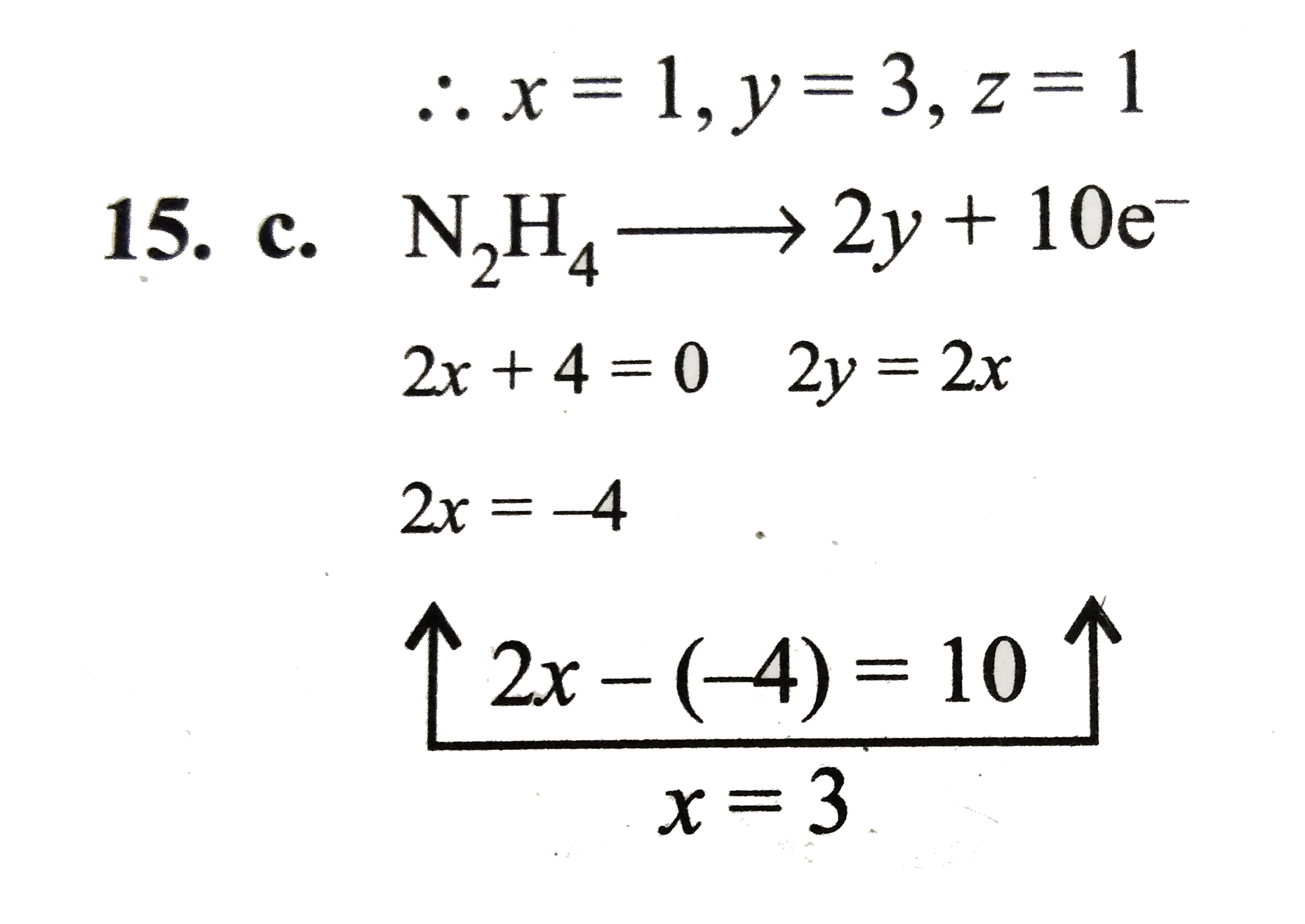A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
REDOX REACTIONS
CENGAGE CHEMISTRY ENGLISH|Exercise Exercises (Assertion-Reasoning)|15 VideosREDOX REACTIONS
CENGAGE CHEMISTRY ENGLISH|Exercise Exercises (Integers)|11 VideosREDOX REACTIONS
CENGAGE CHEMISTRY ENGLISH|Exercise Exercises (Multiple Correct)|32 VideosPURIFICATION OF ORGANIC COMPOUNDS AND QUALITATIVE AND QUANTITATIVE ANALYSIS
CENGAGE CHEMISTRY ENGLISH|Exercise Assertion Reasoning Type|5 VideosS-BLOCK GROUP 1 - ALKALI METALS
CENGAGE CHEMISTRY ENGLISH|Exercise Archives Subjective|8 Videos
Similar Questions
Explore conceptually related problems
CENGAGE CHEMISTRY ENGLISH-REDOX REACTIONS-Exercises (Single Correct)
- Which of the following is not a redox reaction?
Text Solution
|
- In a chemical reaction, K(2)Cr(2)O(7)+xH(2)SO(4)+ySO(2) to K(2)SO(4)+ ...
Text Solution
|
- A mole of N(2)H(4) loses 10 mol of electrons to form a new compound Y....
Text Solution
|
- When copper is treated with a certain concentration of nitric acid, ni...
Text Solution
|
- In which of the following pairs in there the greatest difference in th...
Text Solution
|
- In the reaction 3Br(2)+6CO(3)^(2-)+3H(2)Orarr5Br^(ө)+BrO(3)^(ө)+6HCO...
Text Solution
|
- In the reaction 2FeCl(3)+H(2)Srarr2FeCl(2)+2HCl+S
Text Solution
|
- The oxidation number of cobalt in K [Co(CO)(4)] is
Text Solution
|
- Which of the following is not a disproprotionation reaction? I. NH(4...
Text Solution
|
- which of the following represent redox reactions? I. Cr(2)O(7)^(2-)+...
Text Solution
|
- In which of the following cases is the oxidation state of N atom wrong...
Text Solution
|
- Question : Which of the following is not a disproprotionation reaction...
Text Solution
|
- The number of moles of K(2)Cr(2)O(7) reduced by one mole of Sn^(2+) i...
Text Solution
|
- Which of the following is redox reaction ?
Text Solution
|
- The oxidation state of Fe in Fe(CO)(5) is
Text Solution
|
- In which of the following pairs is there the greatest difference in th...
Text Solution
|
- [Which of the following is not an intermolecular redox reaction?
Text Solution
|
- The number of moles of KMnO(4) required to oxidise 1 mol of Fe(C(2)O(4...
Text Solution
|
- In the reaction K+O(2)rarrKO(2)
Text Solution
|
- Which of the following is the best description of the behaviour of bro...
Text Solution
|