A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
CHEMICAL EQUILIBRIUM
CENGAGE CHEMISTRY ENGLISH|Exercise Exercises (Multiple Correct)|26 VideosCHEMICAL EQUILIBRIUM
CENGAGE CHEMISTRY ENGLISH|Exercise Exercises (Single Correct)|58 VideosCHEMICAL EQUILIBRIUM
CENGAGE CHEMISTRY ENGLISH|Exercise Exercises (Subjective)|46 VideosCHEMICAL BONDING AND MOLECULAR STRUCTURE
CENGAGE CHEMISTRY ENGLISH|Exercise Archives Subjective|15 VideosCLASSIFICATION AND NOMENCLATURE OF ORGANIC COMPOUNDS
CENGAGE CHEMISTRY ENGLISH|Exercise Analytical and Descriptive Type|3 Videos
Similar Questions
Explore conceptually related problems
CENGAGE CHEMISTRY ENGLISH-CHEMICAL EQUILIBRIUM-Exercises (Linked Comprehensive)
- The relation between K(p) and K(c) is K(p)=K(c)(RT)^(Deltan) unit of K...
Text Solution
|
- If K1 and K2 are the respective equilibrium constants for the two rea...
Text Solution
|
- N(2)(g)+3H(2)(g) hArr 2NH(3)(g), DeltaH^(ɵ)=-22.4 kJ The pressure in...
Text Solution
|
- N(2)(g)+3H(2)(g) hArr 2NH(3)(g), DeltaH^(ɵ)=-22.4 kJ The pressure in...
Text Solution
|
- N(2)(g)+3H(2)(g) hArr 2NH(3)(g), DeltaH^(ɵ)=-22.4 kJ The pressure in...
Text Solution
|
- N(2)(g)+3H(2)(g) hArr 2NH(3)(g), DeltaH^(ɵ)=-22.4 kJ The pressure in...
Text Solution
|
- Mass action rato or reaction quotient Q for a reaction can be calculat...
Text Solution
|
- Mass action rato or reaction quotient Q for a reaction can be calculat...
Text Solution
|
- Mass action rato or reaction quotient Q for a reaction can be calculat...
Text Solution
|
- Mass action rato or reaction quotient Q for a reaction can be calculat...
Text Solution
|
- Mass action rato or reaction quotient Q for a reaction can be calculat...
Text Solution
|
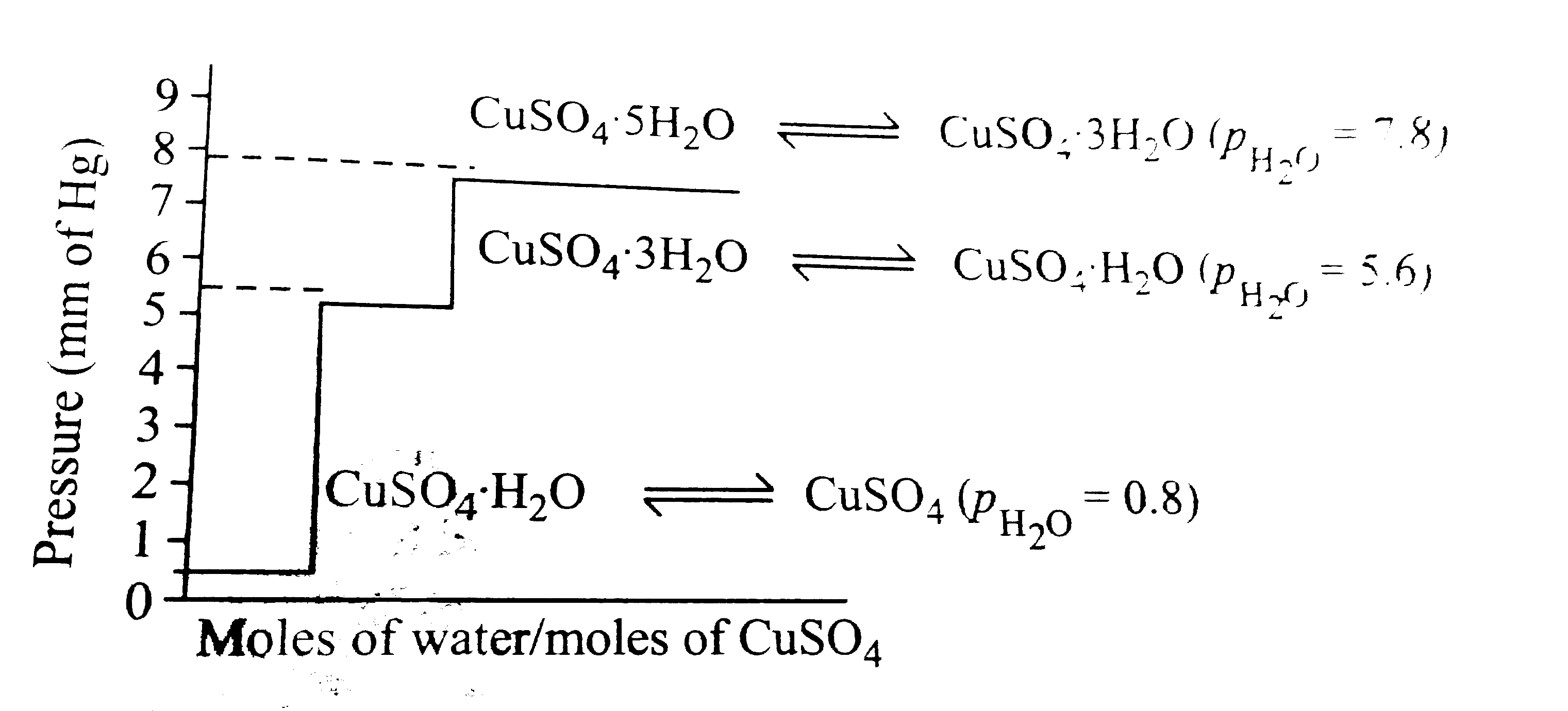
- Dehydration of salts is an important class of heterogeneous reactions....
Text Solution
|
- Dehydration of salts is an important class of heterogeneous reactions....
Text Solution
|
- Dehydration of salts is an important class of heterogeneous reactions....
Text Solution
|
- X, Y and Z react in the 1:1:1 stoichiometric ratio. The concentratio...
Text Solution
|
- X, Y and Z react in the 1:1:1 stoichiometric ratio. The concentratio...
Text Solution
|
- X, Y and Z react in the 1:1:1 stoichiometric ratio. The concentratio...
Text Solution
|
- Two solids X and Y dissociate into gaseous products at a certain tempe...
Text Solution
|
- Two solid X and Y dissociate into gaseous products at a certain temper...
Text Solution
|
- Two solid X and Y dissociate into gaseous products at a certain temper...
Text Solution
|