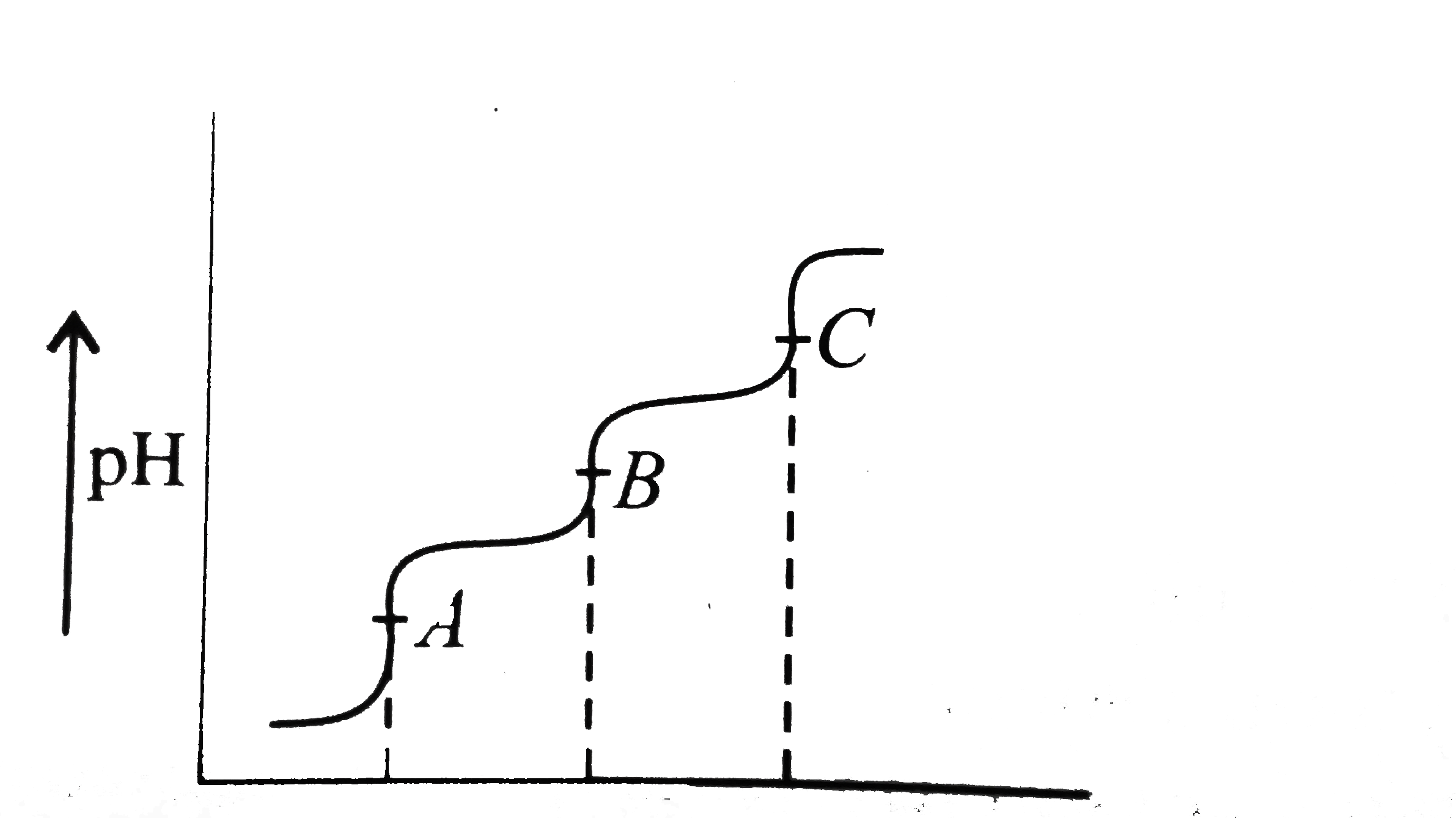
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
IONIC EQUILIBRIUM
CENGAGE CHEMISTRY ENGLISH|Exercise Exercises Multiple Correct|33 VideosIONIC EQUILIBRIUM
CENGAGE CHEMISTRY ENGLISH|Exercise Exercises Single Correct|121 VideosIONIC EQUILIBRIUM
CENGAGE CHEMISTRY ENGLISH|Exercise Exercises Subjective(Coordination Equilibria)|2 VideosHYDROGEN, WATER AND HYDROGEN PEROXIDE
CENGAGE CHEMISTRY ENGLISH|Exercise Subjective Archive (Subjective)|3 VideosISOMERISM
CENGAGE CHEMISTRY ENGLISH|Exercise Assertion-Reasoning Type|1 Videos
Similar Questions
Explore conceptually related problems
CENGAGE CHEMISTRY ENGLISH-IONIC EQUILIBRIUM-Exercises Linked Comprehension
- If methyl orange is used as a solution at pH = 2 containing the indic...
Text Solution
|
- Acidic solution is defined as a solution whose [H^(o+)] gt [overset(Th...
Text Solution
|
- Acidic solution is defined as a solution whose [H^(o+)] gt [overset(Th...
Text Solution
|
- Acidic solution is defined as a solution whose [H^(o+)] gt [overset(Th...
Text Solution
|
- In equalitative analysis, cations of graph II as well as group IV both...
Text Solution
|
- In equalitative analysis, cations of graph II as well as group IV both...
Text Solution
|
- In equalitative analysis, cations of graph II as well as group IV both...
Text Solution
|
- In equalitative analysis, cations of graph II as well as group IV both...
Text Solution
|
- The degree of dissociation of weak electrolyde is inversely proportion...
Text Solution
|
- The degree of dissociation of weak electrolyde is inversely proportion...
Text Solution
|
- The degree of dissociation of weak electrolyde is inversely proportion...
Text Solution
|
- The following solutions are mixed: 500mL of 0.01 M AgNO(3) and 500mL s...
Text Solution
|
- The following solutions are mixed: 500mL of 0.01 M AgNO(3) and 500mL s...
Text Solution
|
- The following solutions are mixed: 500mL of 0.01 M AgNO(3) and 500mL s...
Text Solution
|
- When 1.5mol of CuCI(2).2H(2)O is dissolved in enough water to make 1.0...
Text Solution
|
- When 1.5mol of CuCI(2).2H(2)O is dissolved in enough water to make 1.0...
Text Solution
|
- When 1.5mol of CuCI(2).2H(2)O is dissolved in enough water to make 1.0...
Text Solution
|
- Acid rain takes place dur to combination of acidic oxides with water a...
Text Solution
|
- Acid rain takes place dur to combination of acidic oxides with water a...
Text Solution
|
- In atmosphere, SO(2) and NO are oxidised to SO(3) and NO(2), respectiv...
Text Solution
|