Text Solution
Verified by Experts
Topper's Solved these Questions
NCERT BASED EXERCISE
CENGAGE CHEMISTRY ENGLISH|Exercise Atomic Structure|94 VideosNCERT BASED EXERCISE
CENGAGE CHEMISTRY ENGLISH|Exercise Thermodynamics|50 VideosNCERT BASED EXERCISE
CENGAGE CHEMISTRY ENGLISH|Exercise Chemical Equilibrium|73 VideosISOMERISM
CENGAGE CHEMISTRY ENGLISH|Exercise Assertion-Reasoning Type|1 VideosORGANIC REACTION MECHANISM
CENGAGE CHEMISTRY ENGLISH|Exercise Analytical and Descriptive|6 Videos
Similar Questions
Explore conceptually related problems
CENGAGE CHEMISTRY ENGLISH-NCERT BASED EXERCISE-Redox Reaction
- Justify that the following reactions are redox reactions: (a) CuO(s...
Text Solution
|
- Fluorine reacts with ice and results in the change: H(2)O(s) + F(2)...
Text Solution
|
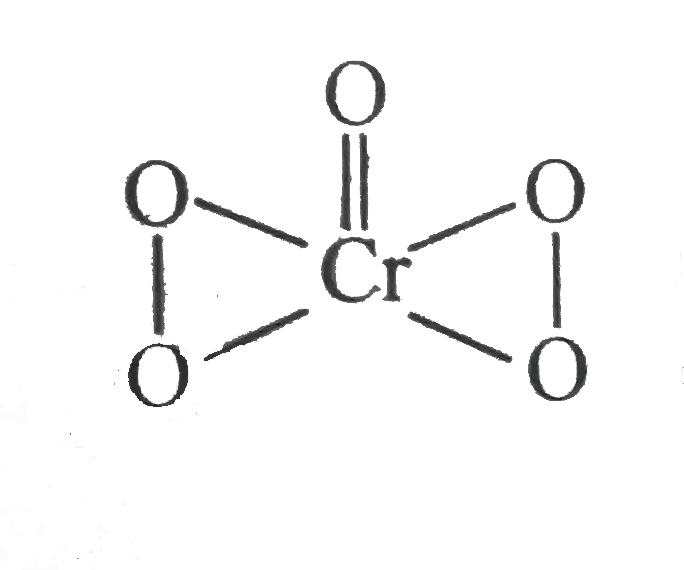
- Calculate the oxidation number of sulphur , chromium and nitrogen in H...
Text Solution
|
- Write the formula for the following compounds: (a) Mercury (II) chlo...
Text Solution
|
- Suggest a list of the substances where carbon can exhibit oxidation st...
Text Solution
|
- While sulphur dioxide and hydrogen peroxide can act as oxidising as we...
Text Solution
|
- Consider the reactions : (a) 6 CO(2) (g) + 6 H(2)O (1) to C(6) H(12)...
Text Solution
|
- The compound AgF(2) is unstable compound. However, if formed, the comp...
Text Solution
|
- Whenever a reaction between an oxidising agent and a reducing agent is...
Text Solution
|
- How do you account for the following observations ? Though alkaline ...
Text Solution
|
- Identify the substance oxidised and reduced, oxidising agent and reduc...
Text Solution
|
- Consider the reactions : 2 S(2)O(3)^(2-) (aq) I(2) (s) to S(4) O(6)^...
Text Solution
|
- Justify giving reactions that among halogens, fluorine is the best oxi...
Text Solution
|
- Why does the following reaction occur ? XeO(6)^(4-) (aq) + 2 F^(-) (...
Text Solution
|
- Consider the reactions : (a) H(3)PO(2) (aq) + 4 AgNO(3) (aq) + 2 H...
Text Solution
|
- Balance the following redox reactions by ion-electron method. MnO(4)...
Text Solution
|
- Balance the following equations in basic medium by ion electron method...
Text Solution
|
- What sorts of informations you can draw from the following reaction ? ...
Text Solution
|
- Mn^(3+) ions are unstable in solution and undergo disproportionation t...
Text Solution
|
- Consider the elements : Cs ,Ne , I and F (a) Identify the element...
Text Solution
|