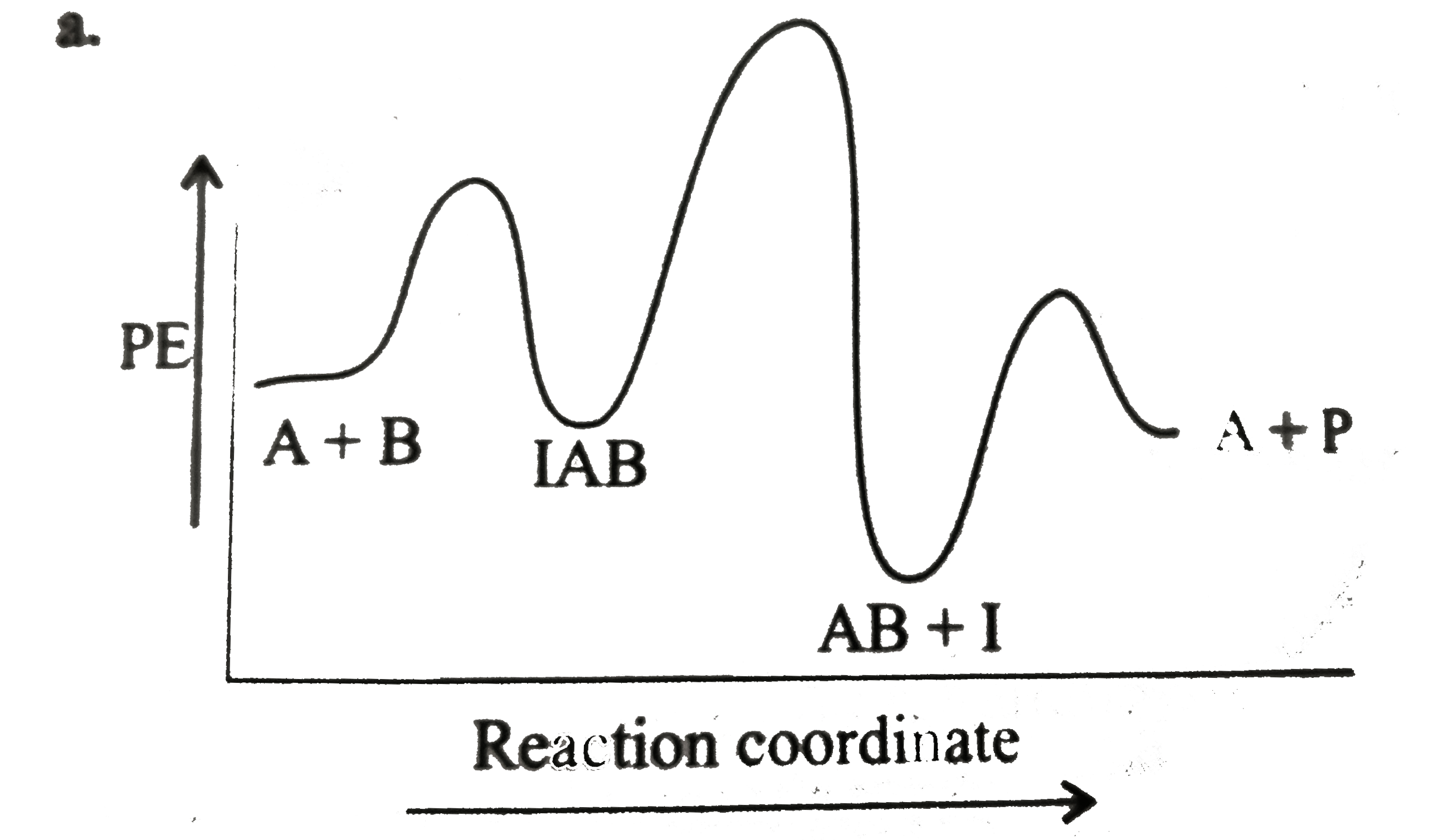
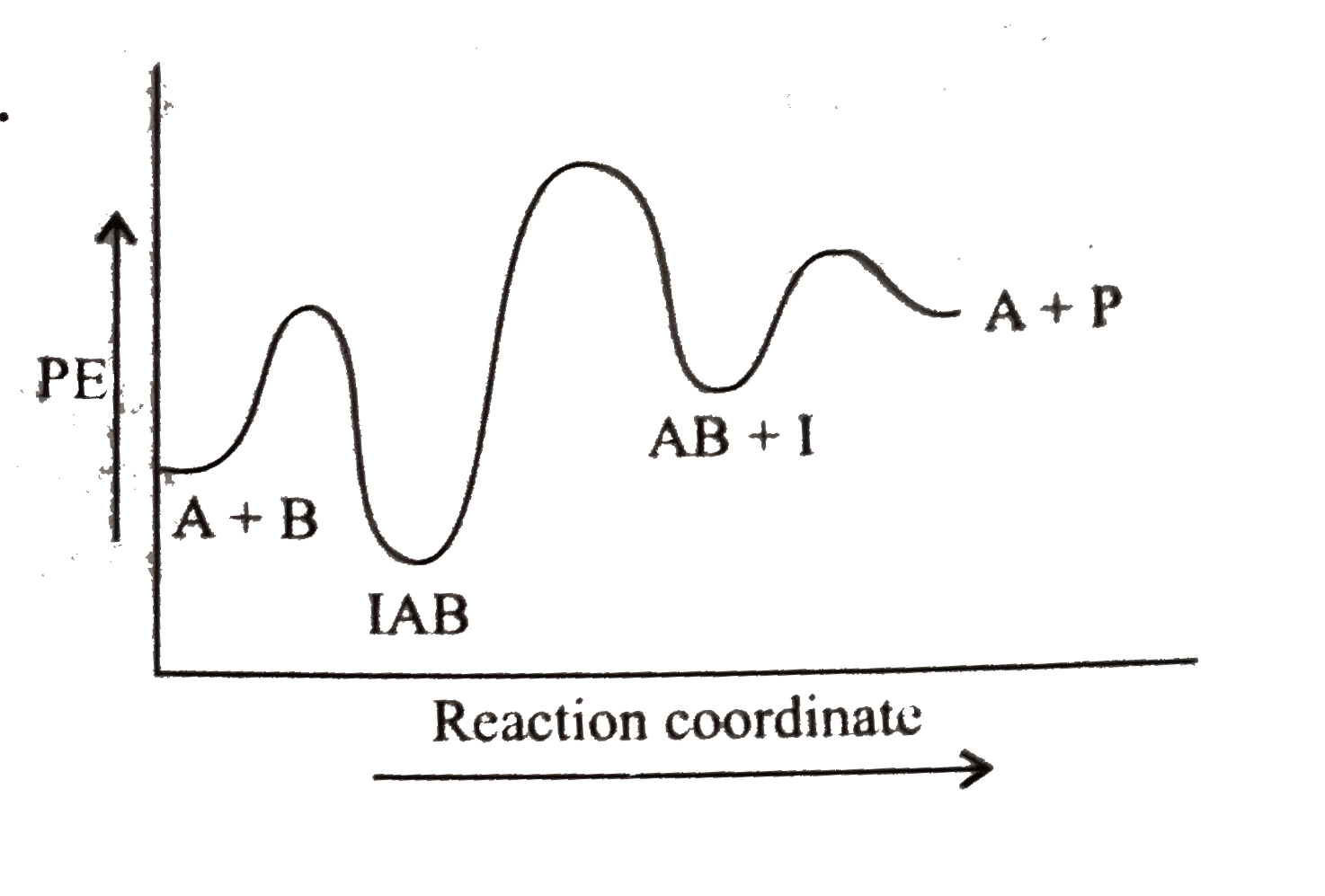
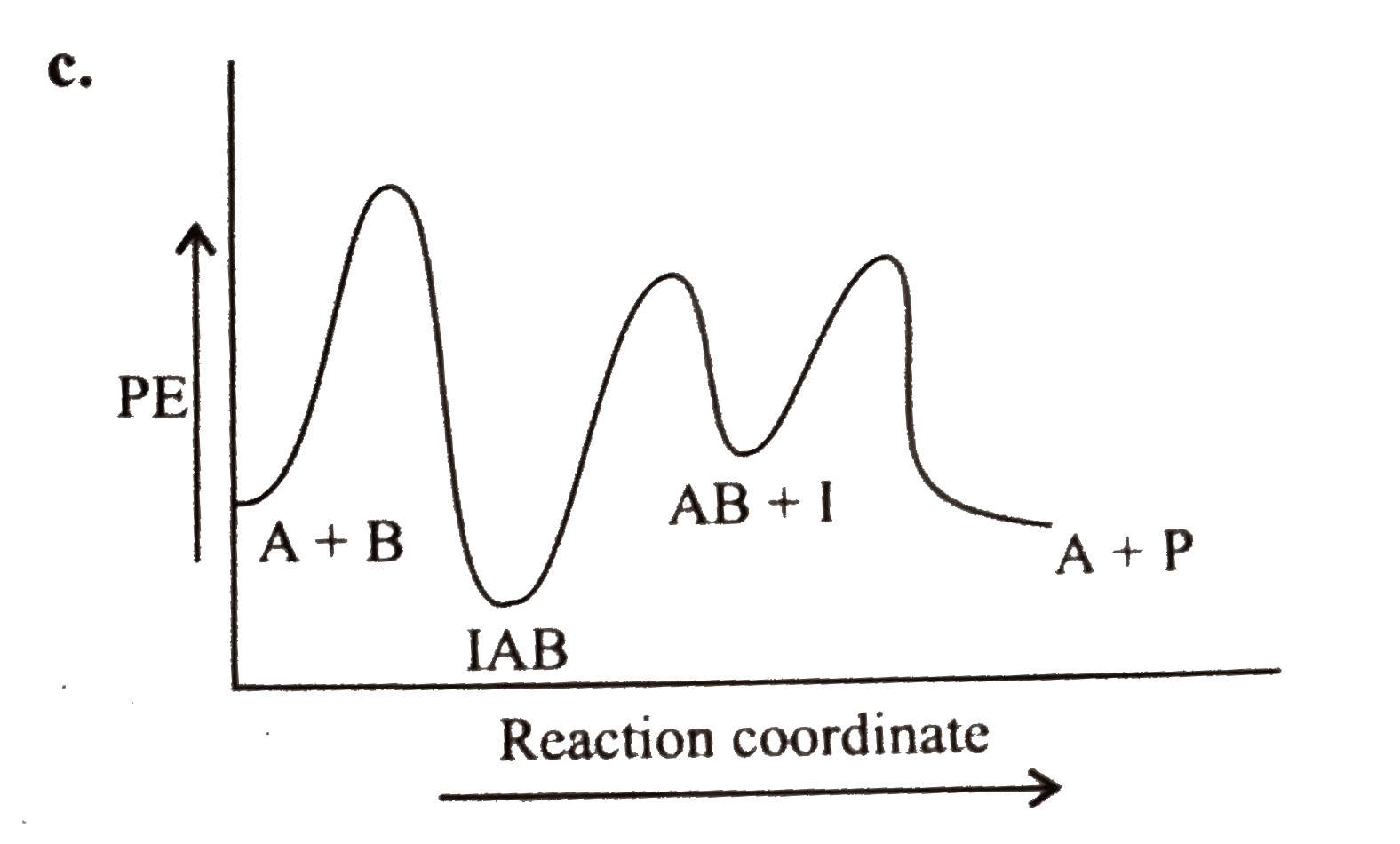
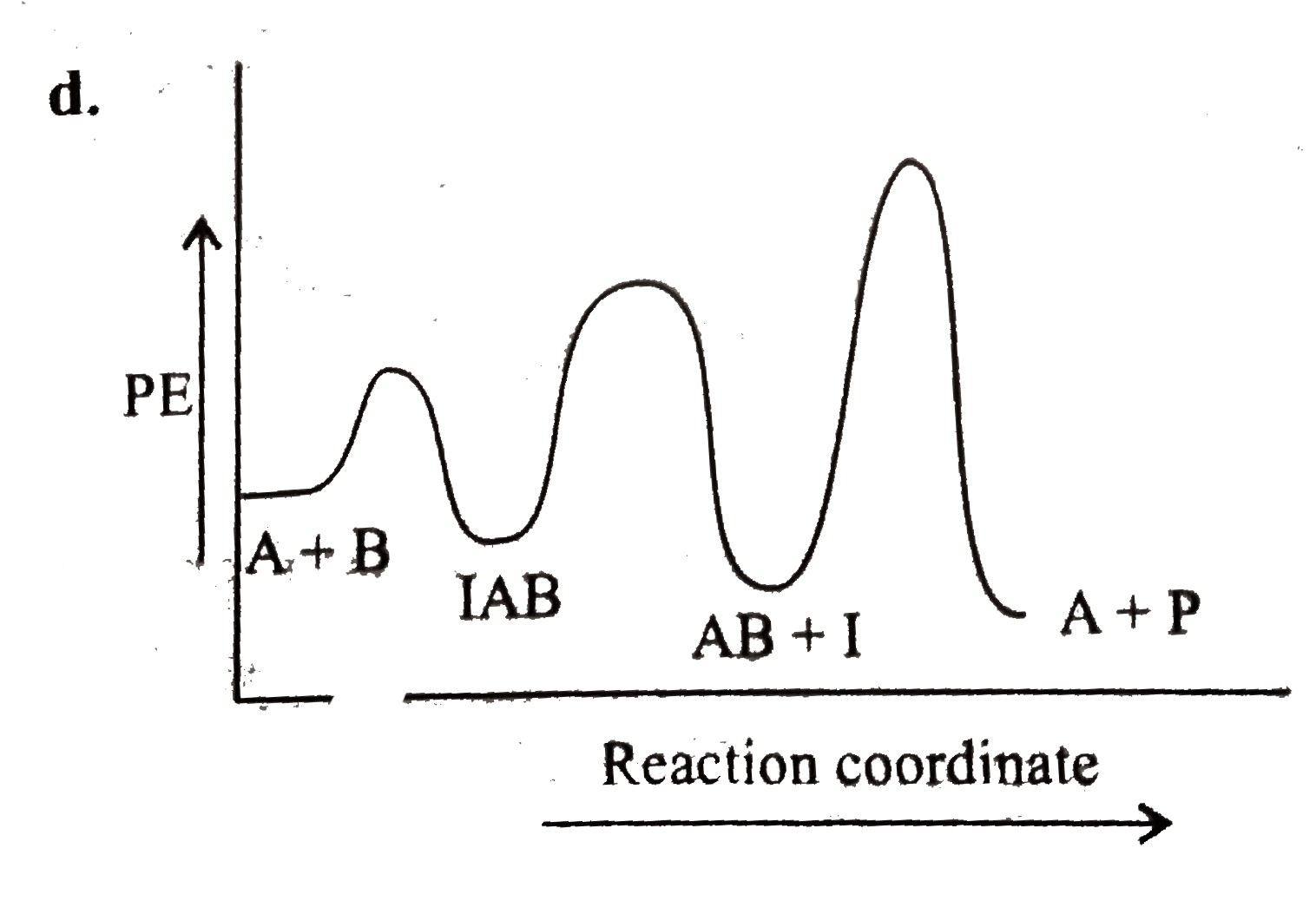
A
B
C
D
Text Solution
AI Generated Solution
The correct Answer is:
Topper's Solved these Questions
CHEMICAL KINETICS
CENGAGE CHEMISTRY ENGLISH|Exercise Ex 4.3 (Objective)|15 VideosCHEMICAL KINETICS
CENGAGE CHEMISTRY ENGLISH|Exercise Ex 4.3 More Than One Correct|5 VideosCHEMICAL KINETICS
CENGAGE CHEMISTRY ENGLISH|Exercise Ex 4.1 (Objective)|9 VideosCARBOXYLIC ACIDS AND THEIR DERIVATIVES
CENGAGE CHEMISTRY ENGLISH|Exercise Exercises Archives (Analytical And Descriptive)|34 VideosCOORDINATION COMPOUNDS
CENGAGE CHEMISTRY ENGLISH|Exercise Archives Subjective|18 Videos
Similar Questions
Explore conceptually related problems
CENGAGE CHEMISTRY ENGLISH-CHEMICAL KINETICS-Ex 4.2 (Objective)
- The reaction A(g) + 2B(g) rarr C(g) + D(g) is an elementary process. I...
Text Solution
|
- In the decompoistion of N(2)O(5)(g), 2N(2)O(5)(g)overset(k(obs))rarr...
Text Solution
|
- Conisder the reaction mechanism: A(2) overset(k(eq))hArr 2A ("fast")...
Text Solution
|
- In the formation of HBr form H(2) and Br(2), following mechanism is ob...
Text Solution
|
- The forward reaction rate for the nitric oxide-oxygen reaction 2NO+O...
Text Solution
|
- Conisder a reaction X + Y rarr Products. If the initial concentration ...
Text Solution
|
- The rate law for the reaction O(3) + O rarr 2O(2) is rate = k[O(3)][NO...
Text Solution
|
- The rate of reaction can be increased by (more than one correct)
Text Solution
|
- The reaction: OCl^(ɵ) + I^(ɵ)overset(overset(ɵ)(OH))rarr OI^(ɵ)+Cl^...
Text Solution
|
- The following mechanism has been proposed for the exothermic catalyzed...
Text Solution
|