Text Solution
Verified by Experts
Topper's Solved these Questions
NUCLEAR CHEMISTRY
CENGAGE CHEMISTRY ENGLISH|Exercise Solved Example|18 VideosNUCLEAR CHEMISTRY
CENGAGE CHEMISTRY ENGLISH|Exercise Ex6.1 Objective|15 VideosNCERT BASED EXERCISE
CENGAGE CHEMISTRY ENGLISH|Exercise Nuclear Chemistry (NCERT Exercise)|29 VideosORGANIC COMPOUNDS WITH FUNCTIONAL GROUP
CENGAGE CHEMISTRY ENGLISH|Exercise Archives Analytical And Descriptive|24 Videos
Similar Questions
Explore conceptually related problems
CENGAGE CHEMISTRY ENGLISH-NUCLEAR CHEMISTRY-Archives Subjective
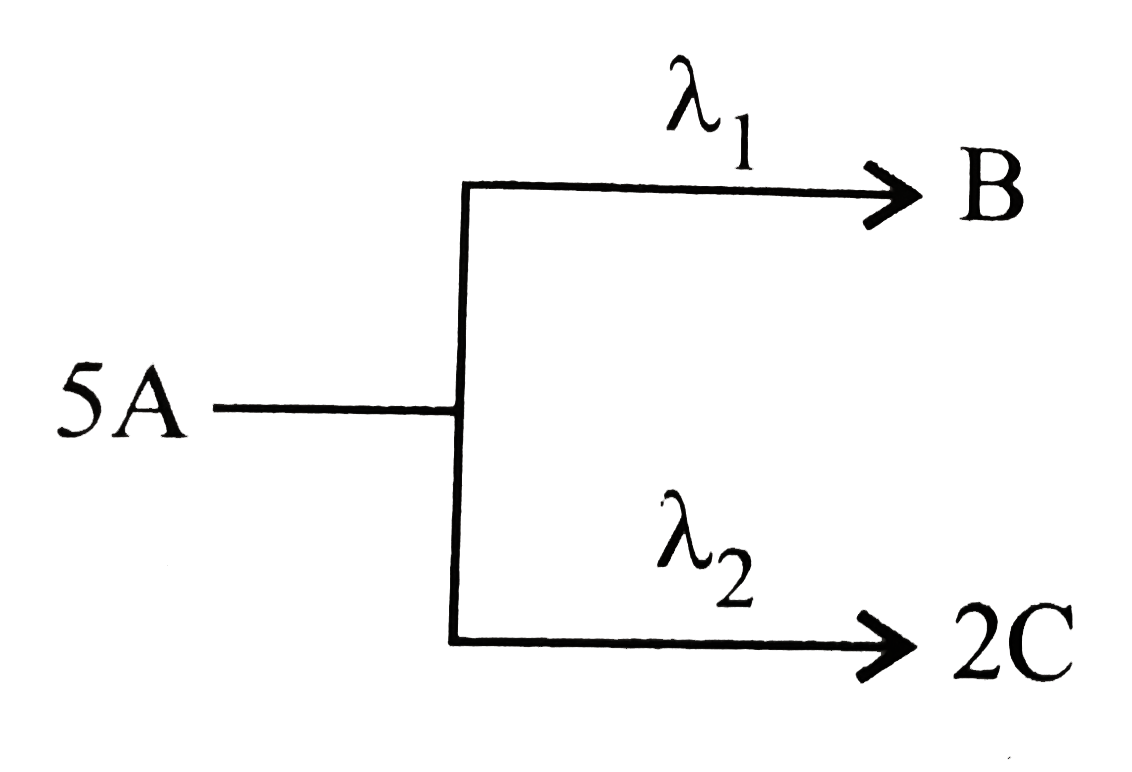
- A follow parallel path of first-order reactions giving B and C as If...
Text Solution
|
- Radioactive decay is a first- order process. Radioactive carbon in woo...
Text Solution
|
- .(90)Th^(234) disintegrates to give .(82)Pb^(206)Pb as the final produ...
Text Solution
|
- An experiment requires minimum beta activity produced at the rate of 3...
Text Solution
|
- The nuclide ratio, .(1)^(3) H to .(1)^(1) H in a sample of water is 8....
Text Solution
|
- One of the hazards of nuclear explosion is the generation of .^(90)Sr ...
Text Solution
|
- Ac^(227) has a half- life of 22.0 years with respect to one leading . ...
Text Solution
|
- Write a balanced equation for the reaction of N^(14) with alpha- parti...
Text Solution
|
- (a) On analysis a sample of uranium ore was found to contain 0.277g of...
Text Solution
|
- By the successive disintegration of ""(92)U^(238), the final product o...
Text Solution
|
- Cu^(64)(" half life" =12.8 "hours" ) decay by beta^(c-)- emission (38%...
Text Solution
|
- Th^(234) disintegrates and emits 6beta- and 7alpha- particles to form...
Text Solution
|
- By the successive disintegration of ""(92)U^(238), the final product o...
Text Solution
|
- Calculate the number of neutrons emitted when .(92)U^(235) undergoes c...
Text Solution
|