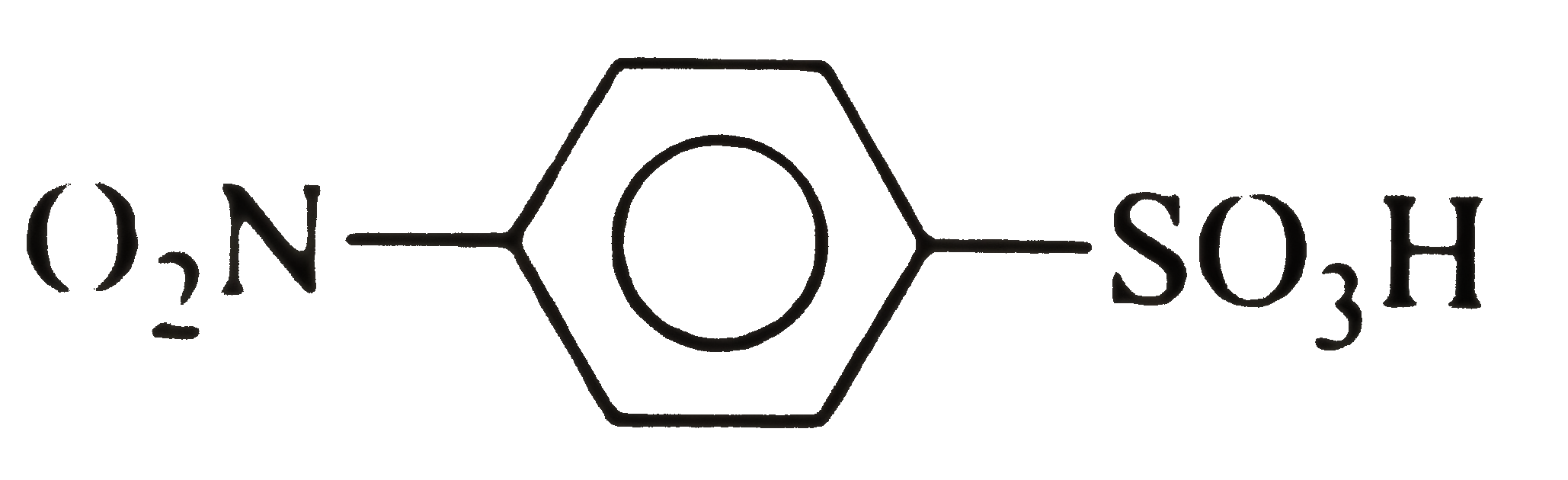
A
B
C
D
Text Solution
AI Generated Solution
The correct Answer is:
Topper's Solved these Questions
PURIFICATION OF ORGANIC COMPOUNDS AND QUALITATIVE AND QUANTITATIVE ANALYSIS
CENGAGE CHEMISTRY ENGLISH|Exercise Multiple Correct Answers Type|10 VideosPURIFICATION OF ORGANIC COMPOUNDS AND QUALITATIVE AND QUANTITATIVE ANALYSIS
CENGAGE CHEMISTRY ENGLISH|Exercise Single Correct Answer Type|40 VideosPURIFICATION OF ORGANIC COMPOUNDS AND QUALITATIVE AND QUANTITATIVE ANALYSIS
CENGAGE CHEMISTRY ENGLISH|Exercise Concept Application Type|23 VideosPERIODIC CLASSIFICATION OF ELEMENTS AND GENERAL INORGANIC CHEMISTRY
CENGAGE CHEMISTRY ENGLISH|Exercise Exercises (Archives )Subjective|4 VideosREDOX REACTIONS
CENGAGE CHEMISTRY ENGLISH|Exercise Archives (Integers)|1 Videos
Similar Questions
Explore conceptually related problems
CENGAGE CHEMISTRY ENGLISH-PURIFICATION OF ORGANIC COMPOUNDS AND QUALITATIVE AND QUANTITATIVE ANALYSIS-Linked Comprehension Type
- Qualitative analysis of organic compounds is performed by Lassaigne's ...
Text Solution
|
- Qualitative analysis of organic compounds is performed by Lassaigne's ...
Text Solution
|
- Qualitative analysis of organic compounds is performed by Lassaigne's ...
Text Solution
|
- Qualitative analysis of organic compounds is performed by Lassaigne's ...
Text Solution
|
- Qualitative analysis of organic compounds is performed by Lassaigne's ...
Text Solution
|
- Lassaigne's extract is boiled with dil. HNO(3) before testing for halo...
Text Solution
|
- Qualitative analysis of organic compounds is performed by Lassaigne's ...
Text Solution
|
- Qualitative analysis of organic compounds is performed by Lassaigne's ...
Text Solution
|
- Qualitative analysis of organic compounds is performed by Lassaigne's ...
Text Solution
|
- Qualitative analysis of organic compounds is performed by Lassaigne's ...
Text Solution
|
- Quantitative estimatin of C,H and extra elements (e.g., N.S.P., and ha...
Text Solution
|
- Quantitative estimatin of C,H and extra elements (e.g., N.S.P., and ha...
Text Solution
|
- Quantitative estimatin of C,H and extra elements (e.g., N.S.P., and ha...
Text Solution
|
- Quantitative estimatin of C,H and extra elements (e.g., N.S.P., and ha...
Text Solution
|
- Quantitative estimatin of C,H and extra elements (e.g., N.S.P., and ha...
Text Solution
|
- Quantitative estimatin of C,H and extra elements (e.g., N.S.P., and ha...
Text Solution
|
- Quantitative estimatin of C,H and extra elements (e.g., N.S.P., and ha...
Text Solution
|
- Quantitative estimatin of C,H and extra elements (e.g., N.S.P., and ha...
Text Solution
|
- Quantitative estimation of C,H and extra elements (e.g., N.S.P., and h...
Text Solution
|
- Quantitative estimation of C,H and extra elements (e.g., N.S.P., and h...
Text Solution
|