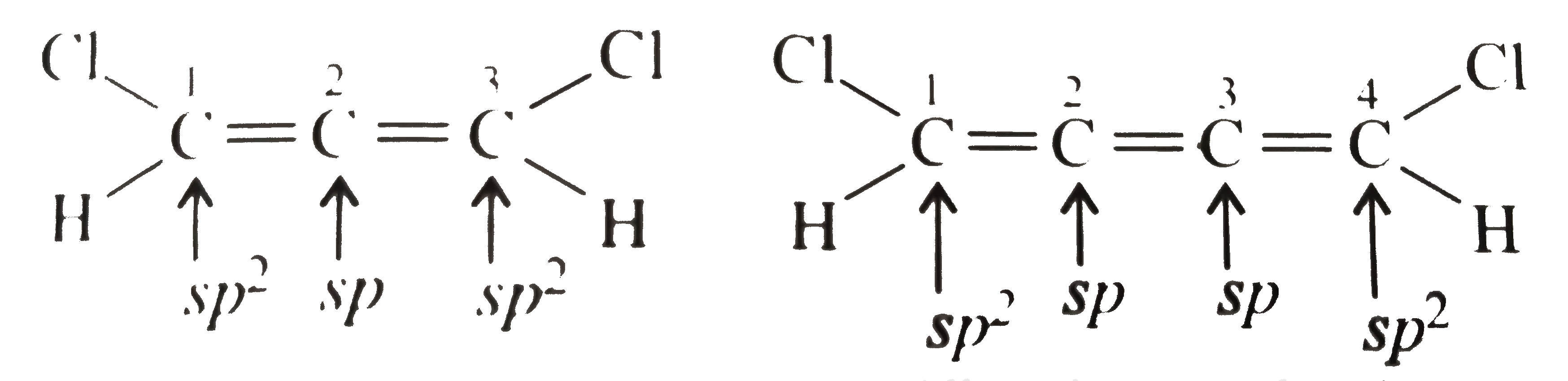
(I) will show dipole moment `(mu)`. The terminal `C` atoms are not in plane with middle `C` atom.
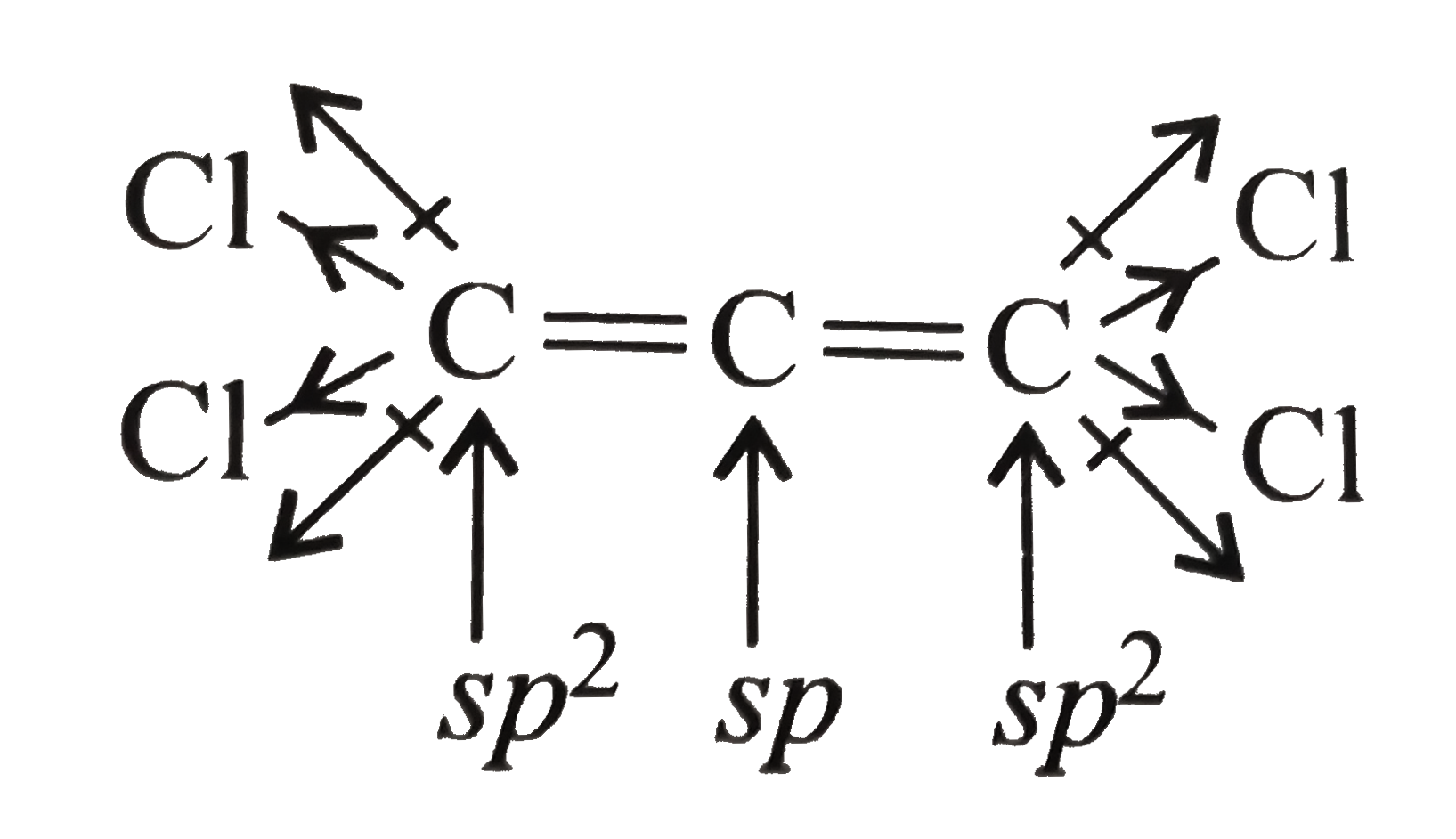
So they have net resultant dipole moment.
(II) has zero dipole moment. All the `C` atoms are in one plane, so the net resultant bond moment is cancelled.
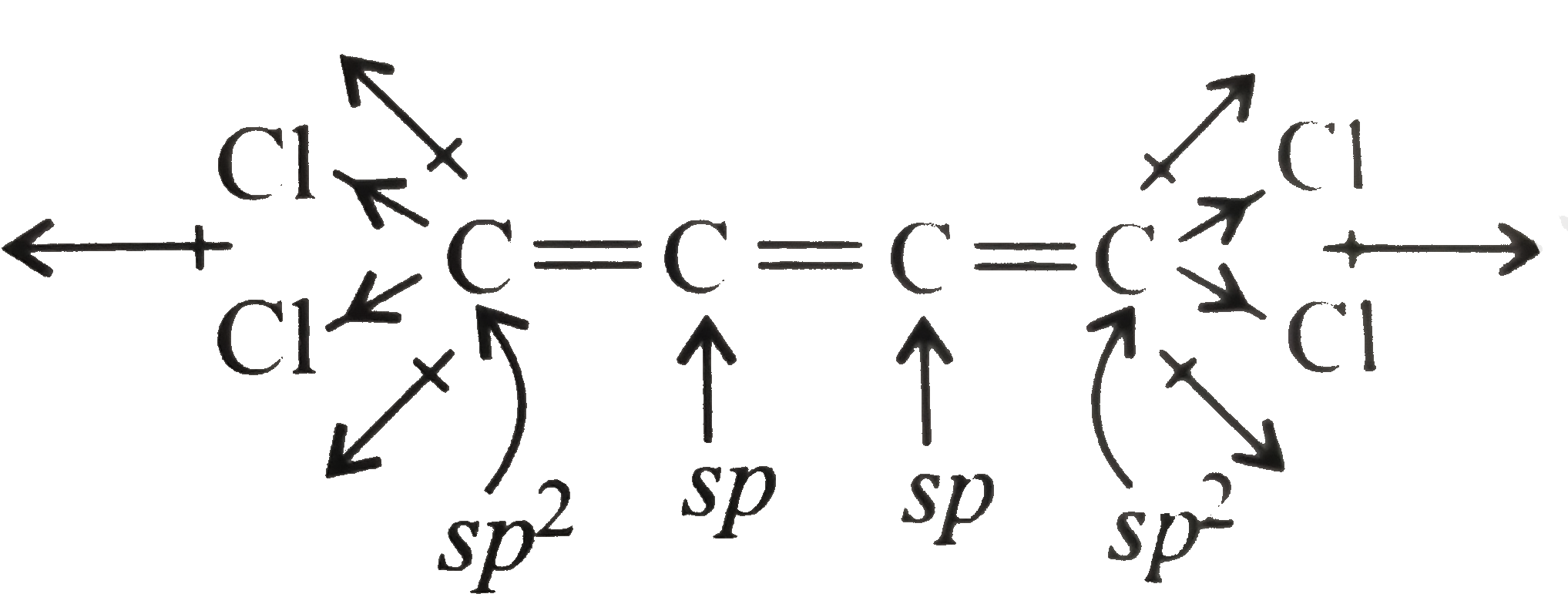
As a general rule, an even number of cumulative double bonds with all the same four groups will show dipole moment and an odd number of cumulative double bonds with all the same four groups will not show dipole moment.
Similarly, an even number of cumulative double bonds with two different groups on terminal `C` atoms will be optically active and an odd number of cumulative double bonds with two different groups on terminal `C` atoms will be optically inactive.