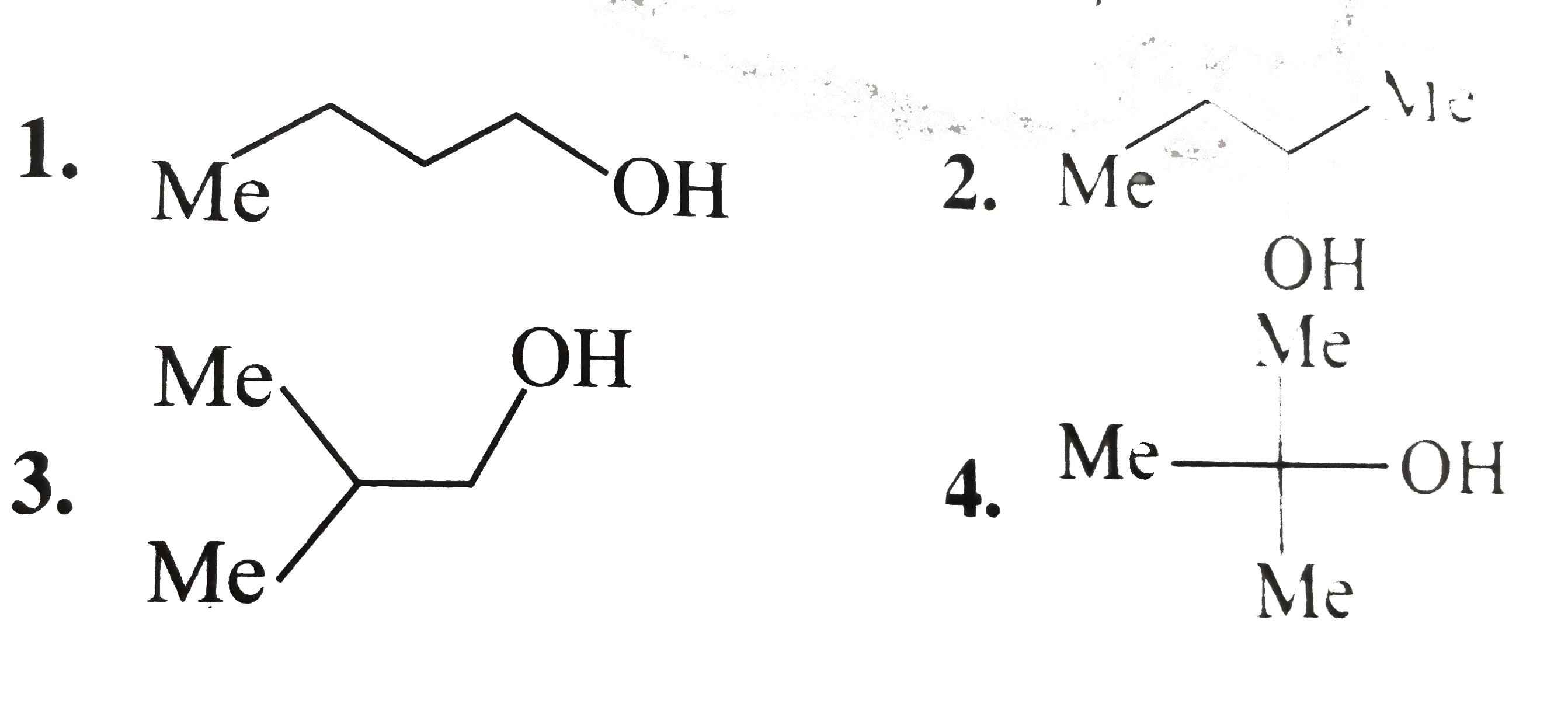
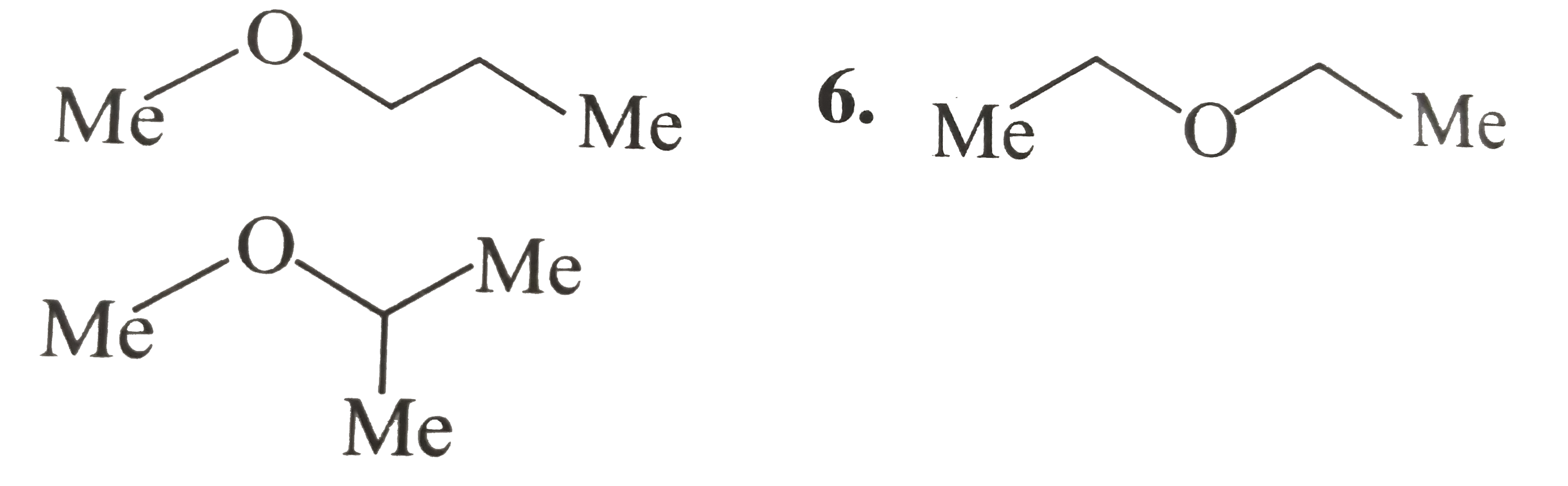A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
ISOMERISM
CENGAGE CHEMISTRY ENGLISH|Exercise Assertion-Reasoning Type (Exercise)|5 VideosISOMERISM
CENGAGE CHEMISTRY ENGLISH|Exercise Fill in the blanks (Exercise)|3 VideosISOMERISM
CENGAGE CHEMISTRY ENGLISH|Exercise Single correct answer type|13 VideosIONIC EQUILIBRIUM
CENGAGE CHEMISTRY ENGLISH|Exercise Archives Subjective|28 VideosNCERT BASED EXERCISE
CENGAGE CHEMISTRY ENGLISH|Exercise Chemical Equilibrium|73 Videos
Similar Questions
Explore conceptually related problems
CENGAGE CHEMISTRY ENGLISH-ISOMERISM-Single correct answer type (Exercise)
- Butene when treated with chlorine at about 500^(@)C forms:
Text Solution
|
- Which of the following are diastereomers ?
Text Solution
|
- An organic compound contains 66% C and 13.3% H. Its vapour density is ...
Text Solution
|
- Which of the following statements regarding the concept of resonance i...
Text Solution
|
- The keto form of phenol contains:
Text Solution
|
- Tautomerism is not exhibited by :
Text Solution
|
- The number of isomers of the compound C(2)FClBrl is :
Text Solution
|
- Pure enantiomeric acid+optically active alcohol having chiral C atom r...
Text Solution
|
- Racemic acid + optically active alcohol having chiral C atom rarr? T...
Text Solution
|
- PhCH(2)CH(Br)Phoverset(Alc.KOH)rarrProduct How many product are poss...
Text Solution
|
- Find compound with formula C(4)H(8)Br(2) How many structure of (X) a...
Text Solution
|
- Hydrogenation of the above compound in the presence of poisoned Pd cat...
Text Solution
|
- Hydrogenation of the above compound in the presence of poisoned Pd cat...
Text Solution
|
- An S(N)2 reaction at an asymmetric carbon of a compound always g...
Text Solution
|
- The nodal plane in the pi -bond of ethene is located in :
Text Solution
|
- Which of the following compounds exhibits stereoisomerism?
Text Solution
|
- Consider the following reaction CH(3)-underset(D)underset(CH)-unders...
Text Solution
|
- Which of the following hydrocarbons has the lowest dipole moment?
Text Solution
|
- The geometrical isomerism is shown by:
Text Solution
|
- The number of geometrical isomers in CH(3)CH=N-OH is
Text Solution
|