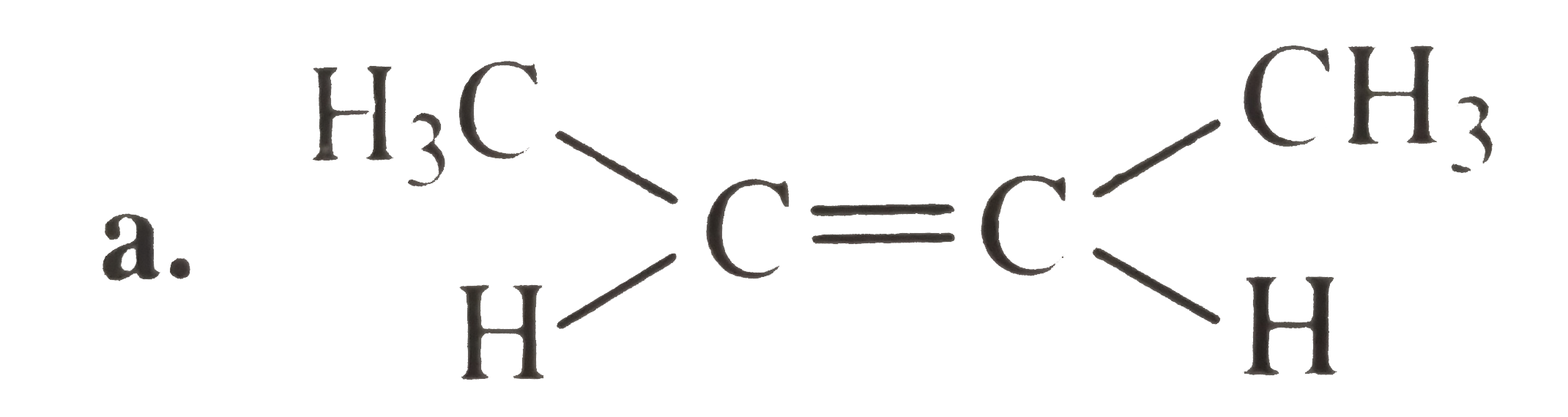A
B
C
D
Text Solution
AI Generated Solution
The correct Answer is:
Topper's Solved these Questions
ISOMERISM
CENGAGE CHEMISTRY ENGLISH|Exercise Assertion-Reasoning Type (Exercise)|5 VideosISOMERISM
CENGAGE CHEMISTRY ENGLISH|Exercise Fill in the blanks (Exercise)|3 VideosISOMERISM
CENGAGE CHEMISTRY ENGLISH|Exercise Single correct answer type|13 VideosIONIC EQUILIBRIUM
CENGAGE CHEMISTRY ENGLISH|Exercise Archives Subjective|28 VideosNCERT BASED EXERCISE
CENGAGE CHEMISTRY ENGLISH|Exercise Chemical Equilibrium|73 Videos
Similar Questions
Explore conceptually related problems
CENGAGE CHEMISTRY ENGLISH-ISOMERISM-Single correct answer type (Exercise)
- Which of the following compounds exhibits stereoisomerism?
Text Solution
|
- Consider the following reaction CH(3)-underset(D)underset(CH)-unders...
Text Solution
|
- Which of the following hydrocarbons has the lowest dipole moment?
Text Solution
|
- The geometrical isomerism is shown by:
Text Solution
|
- The number of geometrical isomers in CH(3)CH=N-OH is
Text Solution
|
- The smallest aldehyde and its next homologue are treated with NH(2)OH ...
Text Solution
|
- What are the numerical values of N and M?
Text Solution
|
- Molecule in which the distance between two adjacent carbon atom is lar...
Text Solution
|
- The compound which is not isomeric with diethyl ether is:
Text Solution
|
- Among the following, the compound that can be most readily sulphonated...
Text Solution
|
- Which of the following compounds will exhibit cis-trans (geometrical) ...
Text Solution
|
- An isomer of ethanol is:
Text Solution
|
- Which of the following will have the least hindered rotation about car...
Text Solution
|
- The number of isomers of C(6)H(14) is:
Text Solution
|
- The enolic form of acetone contains:
Text Solution
|
- Three dimensional arrangements which ca be interconverted into one ano...
Text Solution
|
- How many optically active stereoisomers are possible for butane-2, 3-d...
Text Solution
|
- The optically active tartaric acid is named as D-(+)- tartaric acid be...
Text Solution
|
- Which of the following compounds will exhibit geometrical isomerism ?
Text Solution
|
- The number of isomers of the compound C(2)FClBrl is :
Text Solution
|