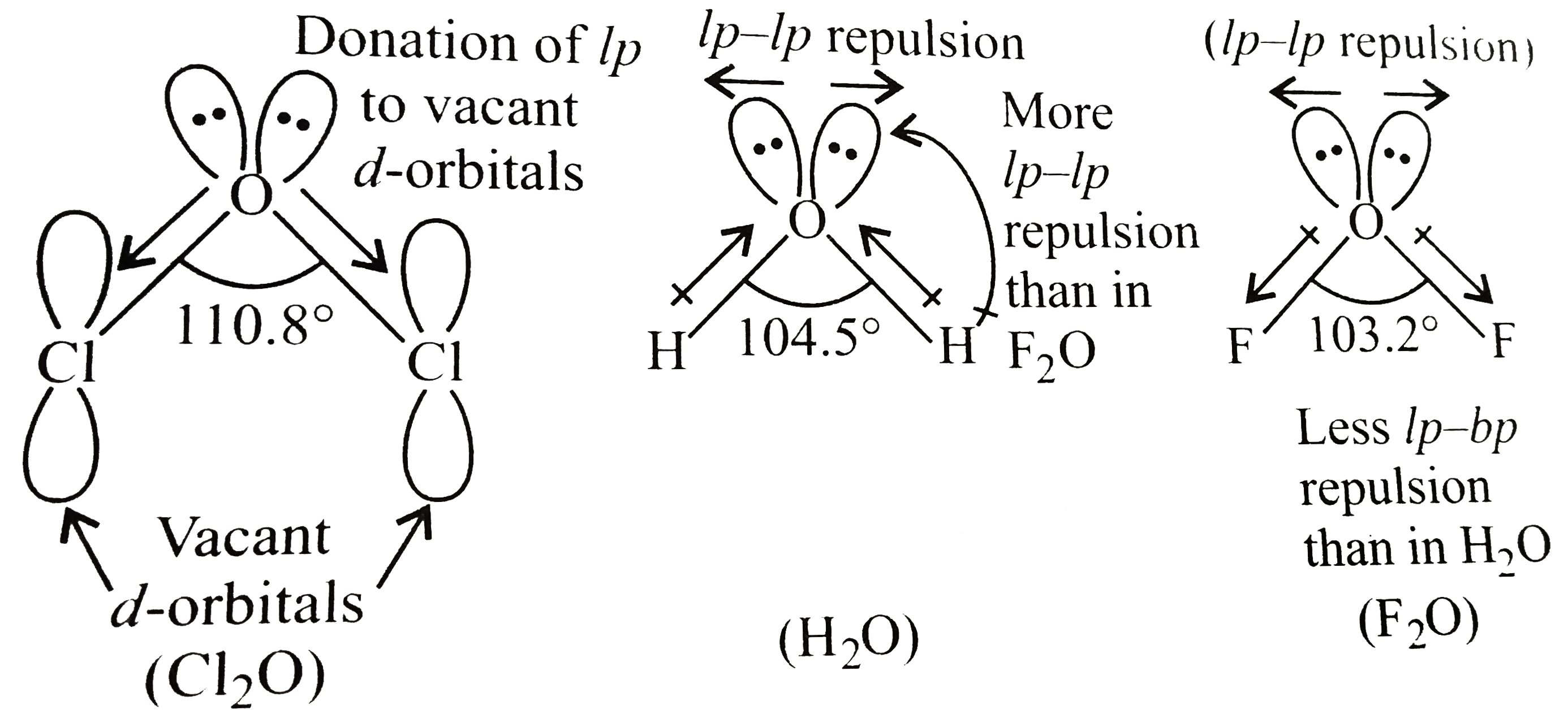Text Solution
Verified by Experts
Topper's Solved these Questions
PERIODIC CLASSIFICATION OF ELEMENTS AND GENERAL INORGANIC CHEMISTRY
CENGAGE CHEMISTRY ENGLISH|Exercise Ex 1.3 (Objective)|8 VideosPERIODIC CLASSIFICATION OF ELEMENTS AND GENERAL INORGANIC CHEMISTRY
CENGAGE CHEMISTRY ENGLISH|Exercise Ex 1.3 (Multiple Correct)|5 VideosPERIODIC CLASSIFICATION OF ELEMENTS AND GENERAL INORGANIC CHEMISTRY
CENGAGE CHEMISTRY ENGLISH|Exercise Ex 1.2(Objective)|22 VideosP-BLOCK GROUP 14 - CARBON FAMILY
CENGAGE CHEMISTRY ENGLISH|Exercise Exercises Archives (Subjective)|9 VideosPURIFICATION OF ORGANIC COMPOUNDS AND QUALITATIVE AND QUANTITATIVE ANALYSIS
CENGAGE CHEMISTRY ENGLISH|Exercise Assertion Reasoning Type|5 Videos
Similar Questions
Explore conceptually related problems
CENGAGE CHEMISTRY ENGLISH-PERIODIC CLASSIFICATION OF ELEMENTS AND GENERAL INORGANIC CHEMISTRY-Ex 1.3
- Arrange the following in order of decreasing ionic character. a. ClF...
Text Solution
|
- Arrange the following in order of decreasing bond angle. CO(2), H(2...
Text Solution
|
- Ca CO(3) dissolves in HCl but not in water. Why ?
Text Solution
|
- Why MgO exist as Mg^(2+)O^(2-) not as Mg^(o+)O^(ɵ) whereas the formati...
Text Solution
|
- Anhydrous AlCl(3) is covalent. From the date given below, predict whet...
Text Solution
|
- Which compound for each of the following pairs is more ionic and why ?...
Text Solution
|
- NaBr gives pale yellow precipitate with AgNO(3) solution but CBr(4) do...
Text Solution
|
- Copper is conducting as such while Cu SO(4) is conducting only in molt...
Text Solution
|
- Explain the observed bond angle order. Cl(2)O(110.8^(@)) gt H(2)O (1...
Text Solution
|
- overset(o+)(NH(4)) has bond angle identical to CH(4) but NH(3) has dif...
Text Solution
|
- Electronegativities of F, O, N, Cl, H are 4.0, 3.5, 3, 3 and 2.1 respe...
Text Solution
|
- The IE(1) of Li is 5.4 eV and IE(1) of H is 13.6 eV. Calculate the ch...
Text Solution
|
- SnCl(2) is solid but SnCl(4) is liquid. Why ?
Text Solution
|