A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
CHEMICAL BONDING AND MOLECULAR STRUCTURE
CENGAGE CHEMISTRY ENGLISH|Exercise Exercises Single Correct (Bond Angle)|10 VideosCHEMICAL BONDING AND MOLECULAR STRUCTURE
CENGAGE CHEMISTRY ENGLISH|Exercise Exercises Single Correct (Resonance And Formal Charges)|10 VideosCHEMICAL BONDING AND MOLECULAR STRUCTURE
CENGAGE CHEMISTRY ENGLISH|Exercise Exercises Single Correct (Dipole Moment)|16 VideosATOMIC STRUCTURE
CENGAGE CHEMISTRY ENGLISH|Exercise Concept Applicationexercise(4.3)|19 VideosCHEMICAL EQUILIBRIUM
CENGAGE CHEMISTRY ENGLISH|Exercise Subjective type|1 Videos
Similar Questions
Explore conceptually related problems
CENGAGE CHEMISTRY ENGLISH-CHEMICAL BONDING AND MOLECULAR STRUCTURE-Exercises Single Correct (Hybridisation)
- The geometry and the type of hybrid orbitals present about the central...
Text Solution
|
- SF(2),SF(4) and SF(6) have the hybridisation at sulphur atom respectiv...
Text Solution
|
- Two types FXF angles are presnet in which of the following molecule (X...
Text Solution
|
- A sigma bonded molecule MX(3) is T-shaped The number of non-bonding pa...
Text Solution
|
- In NH(4)^+ and OF(2) th hybridisation of central atom respectively are...
Text Solution
|
- Hybridisation involves .
Text Solution
|
- As PF(5) molecule is sp^(3) d hybridised and is trigonal bipyramidal (...
Text Solution
|
- If the geometry of [PtCl4]^(2-) -is square planar, which orbitals are ...
Text Solution
|
- SeF(6) is sp^(3)d^(2) hybridised and is octahedral (OH) Which d orbita...
Text Solution
|
- IF(7) is sp^(3)d^(3) hybridised and is (pentagonal bipyramid) Which d ...
Text Solution
|
- In a regular octahedral molecule SF(6) the number of F-S-F bonds at 18...
Text Solution
|
- The maximum number of 90^(@) angles between bp-bp of electrons is obse...
Text Solution
|
- Among the following ions the ppi-dpi overlap could be present in
Text Solution
|
- Which of the following have distored octahedral structure ? .
Text Solution
|
- Sulphur reacts with chlorine in 1:2 ratio and forms X hydrolysis of X ...
Text Solution
|
- Orthonitrophenol is steam volatile but paranitrophenol is not because ...
Text Solution
|
- Which of the following compounds has the least tendency to from H-bond...
Text Solution
|
- Which of the following molecule forms linear polymeric structure due...
Text Solution
|
- Which one of the following hydrogen halides has the lowest boilling po...
Text Solution
|
- Out of the two compounds shown below the vapour pressure of II at a ...
Text Solution
|
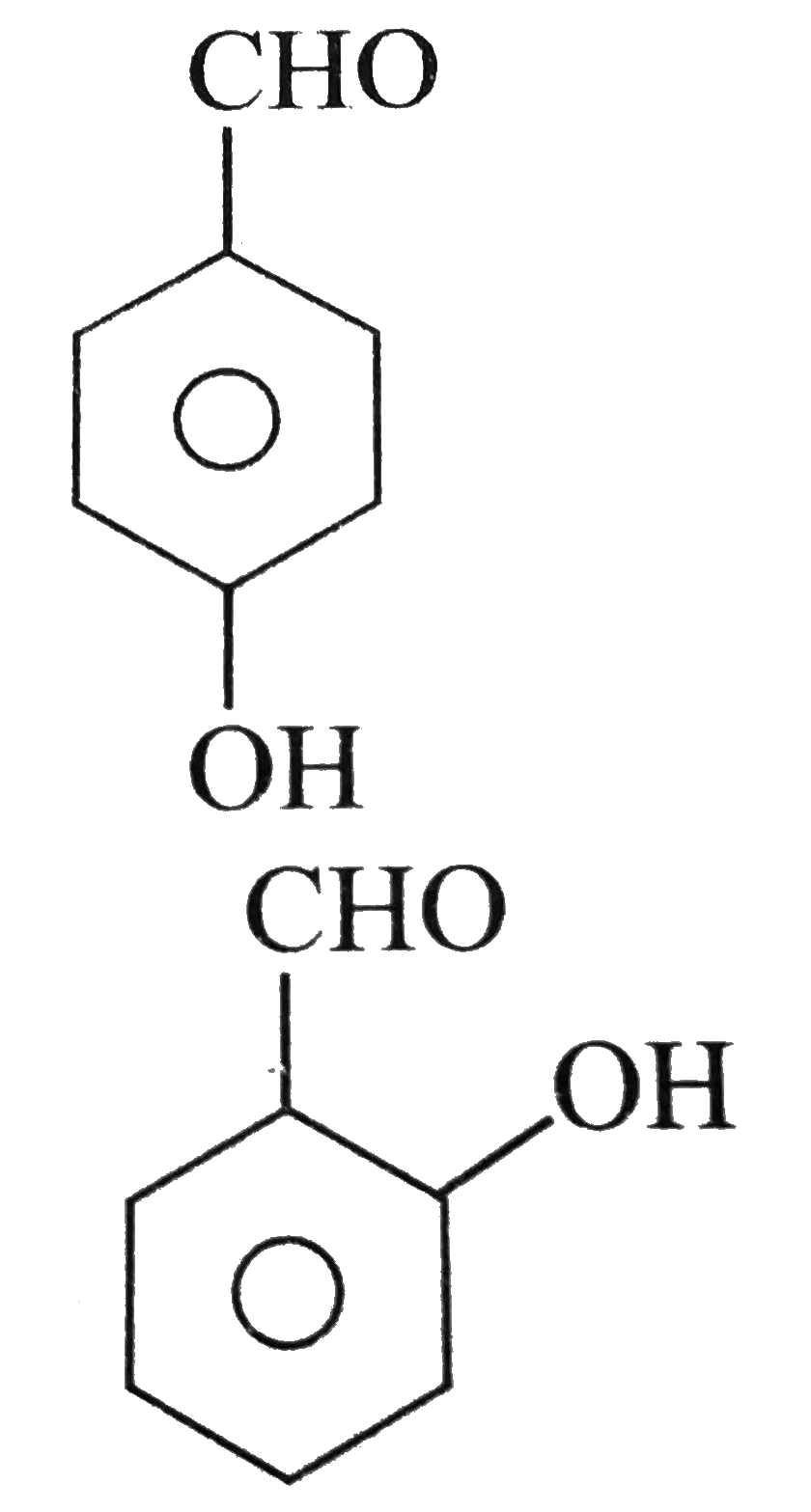 .
.