A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
CHEMICAL BONDING AND MOLECULAR STRUCTURE
CENGAGE CHEMISTRY ENGLISH|Exercise Archives Single Correct|55 VideosCHEMICAL BONDING AND MOLECULAR STRUCTURE
CENGAGE CHEMISTRY ENGLISH|Exercise Archives Integer|2 VideosCHEMICAL BONDING AND MOLECULAR STRUCTURE
CENGAGE CHEMISTRY ENGLISH|Exercise Exercises True/False|20 VideosATOMIC STRUCTURE
CENGAGE CHEMISTRY ENGLISH|Exercise Concept Applicationexercise(4.3)|19 VideosCHEMICAL EQUILIBRIUM
CENGAGE CHEMISTRY ENGLISH|Exercise Subjective type|1 Videos
Similar Questions
Explore conceptually related problems
CENGAGE CHEMISTRY ENGLISH-CHEMICAL BONDING AND MOLECULAR STRUCTURE-Archives Multiple Correct
- CO(2) is isostructural with
Text Solution
|
- The linear struture is assumed by :
Text Solution
|
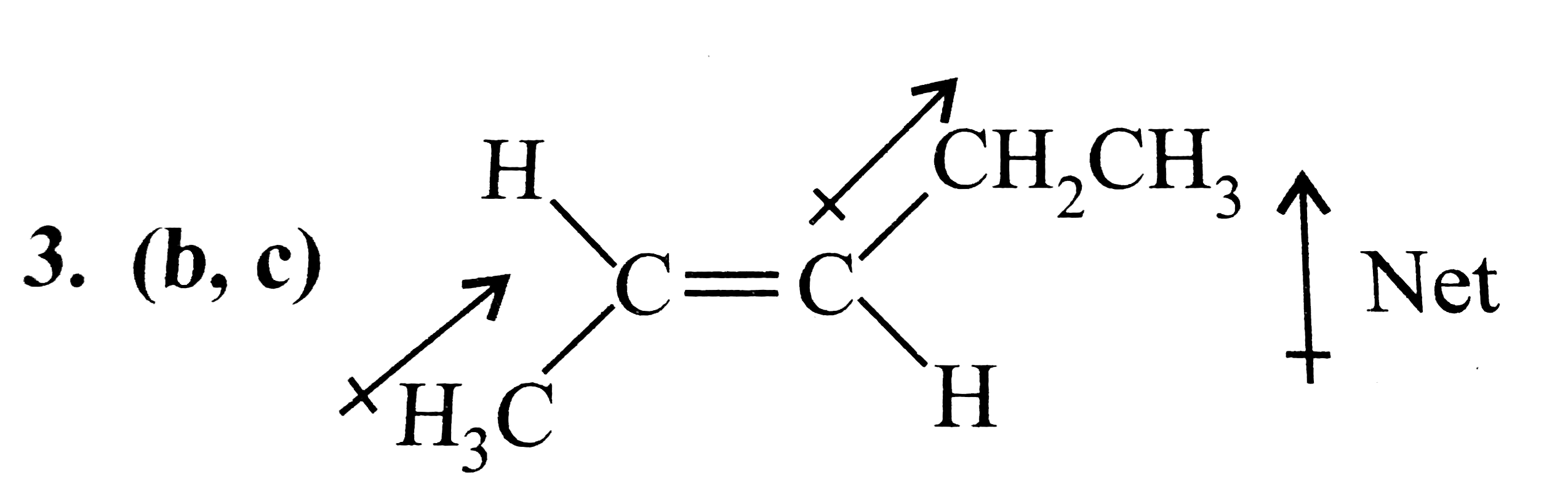
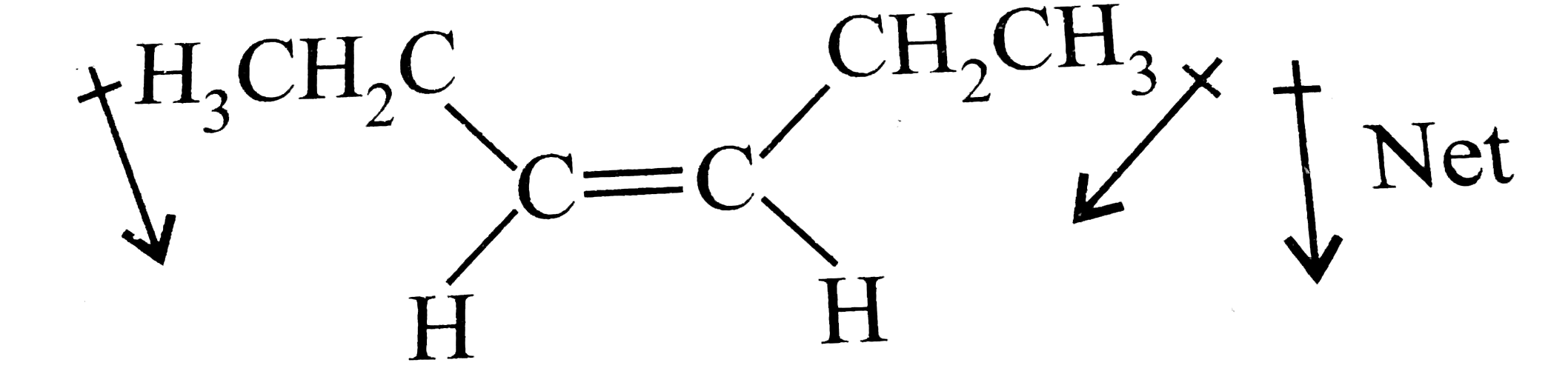
- The molecule (s) that will have dipole moment is/are:
Text Solution
|
- Which of the folowing have identical bond orders ? .
Text Solution
|
- Out of CH(3)^(o+),H(3)O^(o+),NH(3),CH(3)^(Theta) the species which is ...
Text Solution
|
- The critical temperature of water is higher than that of O(2) because ...
Text Solution
|
- The geometry and the type of hybrid orbital present about the central ...
Text Solution
|
- The nitrogen oxide (s) that contain (s) N-N bonds (s) is (are).
Text Solution
|
- Hydrogen bonding plays a central role in which of the following phenom...
Text Solution
|
- When O2 is adsorbed on ametallic surface, electron transfer occurs fro...
Text Solution
|
 .
.