A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
HYDROGEN, WATER AND HYDROGEN PEROXIDE
CENGAGE CHEMISTRY ENGLISH|Exercise Exercises (Assertion Reasoning)|17 VideosHYDROGEN, WATER AND HYDROGEN PEROXIDE
CENGAGE CHEMISTRY ENGLISH|Exercise Exercises (Interger)|10 VideosHYDROGEN, WATER AND HYDROGEN PEROXIDE
CENGAGE CHEMISTRY ENGLISH|Exercise Exercises Multiple Correct|19 VideosGENERAL ORGANIC CHEMISTRY
CENGAGE CHEMISTRY ENGLISH|Exercise Analytical and Descriptive|1 VideosIONIC EQUILIBRIUM
CENGAGE CHEMISTRY ENGLISH|Exercise Archives Subjective|28 Videos
Similar Questions
Explore conceptually related problems
CENGAGE CHEMISTRY ENGLISH-HYDROGEN, WATER AND HYDROGEN PEROXIDE-Exercises (Single Correct)
- A 5.0 mL solution of H(2)O(2) liberates 1.27 g of iodine from an acidi...
Text Solution
|
- 100 mL of ozone (O3) at STP were passed through 100 mL of 10 volum...
Text Solution
|
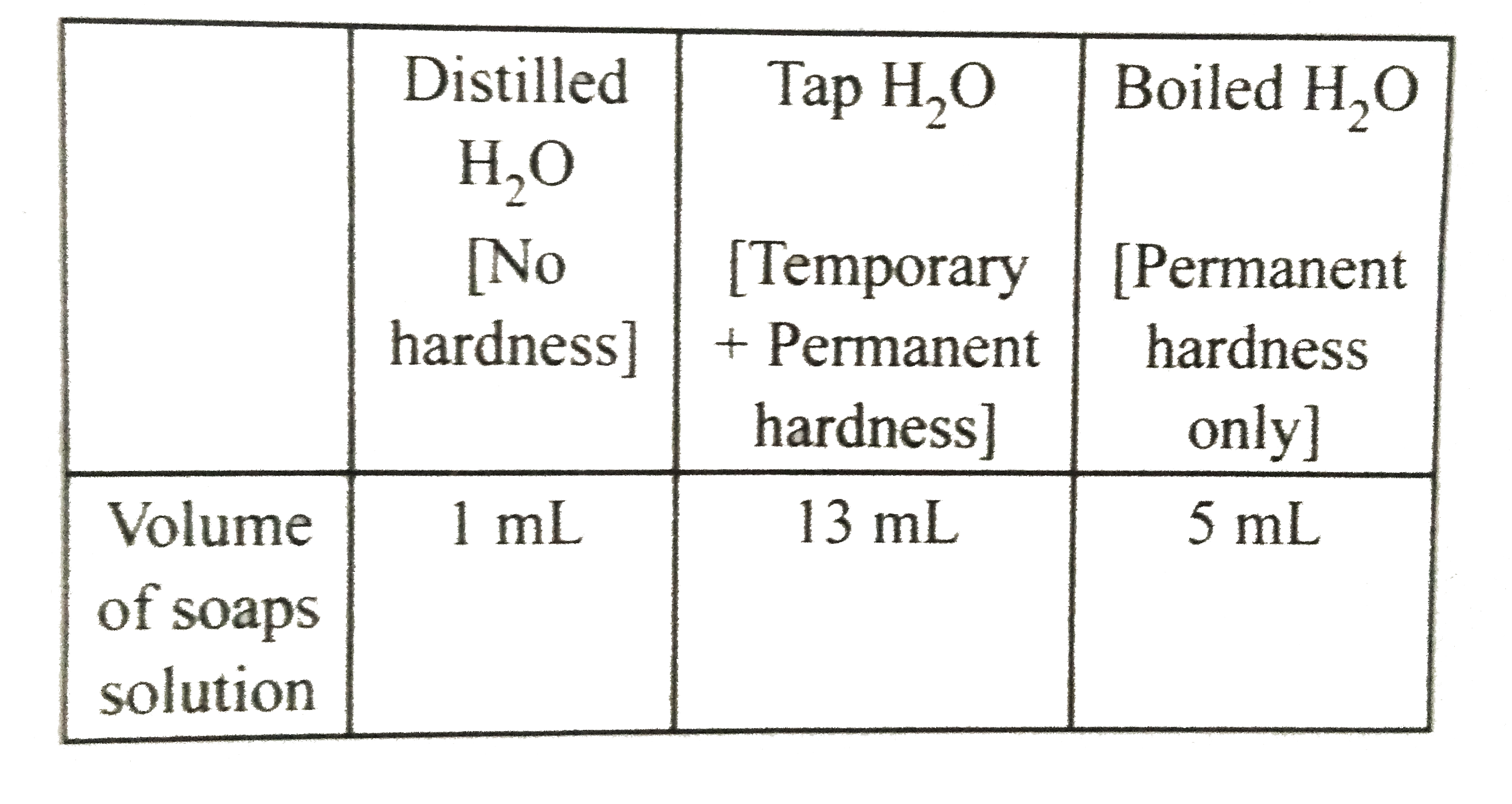
- 25 mL sample of distilled water, tap water and boiled water required, ...
Text Solution
|
- 3.4 g sample of H(2)O(2) solution containing x% H(2)O(2) by weight req...
Text Solution
|
- If 100 mL of acidified 2NH(2)O(2) is allowed to react with KMnO(4) sol...
Text Solution
|
- 100mL of H2O2 is oxidised by 100mL of 0.01M KMnO4 in acidic medium (Mn...
Text Solution
|
- 10mL of H2O2 solution (volume strength = x) requires 10mL of N//0.56 M...
Text Solution
|
- The normality and volume strength of a solution made by mixing 1.0 L...
Text Solution
|
- 100mL of H2O2 is oxidised by 100mL of 0.01M KMnO4 in acidic medium (Mn...
Text Solution
|
- The purity of H2O2 in a given sample is 85%. Calculate the weight of i...
Text Solution
|
- 10 L of hard water required 0.56 g of lime (CaO) for removing hardness...
Text Solution
|
- Hydrogen has the tendency to gain one election to acquire helium confi...
Text Solution
|
- Heavy water is qualified as heavy liquid as it is.
Text Solution
|
- Which of the following is used as rocket fuel?
Text Solution
|
- On burning hydrogen in air the colour of flame is
Text Solution
|
- Number of H-bonds formed by a water molecule is:
Text Solution
|
- Surface water contains.
Text Solution
|
- Which is false about H2O2?
Text Solution
|
- When electric current is passed through an ionic hydride in molten sta...
Text Solution
|
- Among CaH2, NH3, NaH and B2H6 which are covalent hydrides?
Text Solution
|