Text Solution
Verified by Experts
Topper's Solved these Questions
S-BLOCK GROUP 1 - ALKALI METALS
CENGAGE CHEMISTRY ENGLISH|Exercise Ex 4.1 (True/False)|1 VideosS-BLOCK GROUP 1 - ALKALI METALS
CENGAGE CHEMISTRY ENGLISH|Exercise Ex 4.1 (Objective)|17 VideosS-BLOCK GROUP 1 - ALKALI METALS
CENGAGE CHEMISTRY ENGLISH|Exercise Solved Examples|11 VideosREDOX REACTIONS
CENGAGE CHEMISTRY ENGLISH|Exercise Archives (Integers)|1 VideosS-BLOCK GROUP 2 - ALKALINE EARTH METALS
CENGAGE CHEMISTRY ENGLISH|Exercise Ex 5.1 Objective|2 Videos
Similar Questions
Explore conceptually related problems
CENGAGE CHEMISTRY ENGLISH-S-BLOCK GROUP 1 - ALKALI METALS-Ex 4.1 (Subjective)
- Why superoxides of of alkali metals are paramagnetic ?
Text Solution
|
- Alkali metals are paramagnetic but their salts are dimagnetic. Explain...
Text Solution
|
- Give reasons for the following. LiI has lower melting point than LiF...
Text Solution
|
- The Haber process can be represented as follows
Text Solution
|
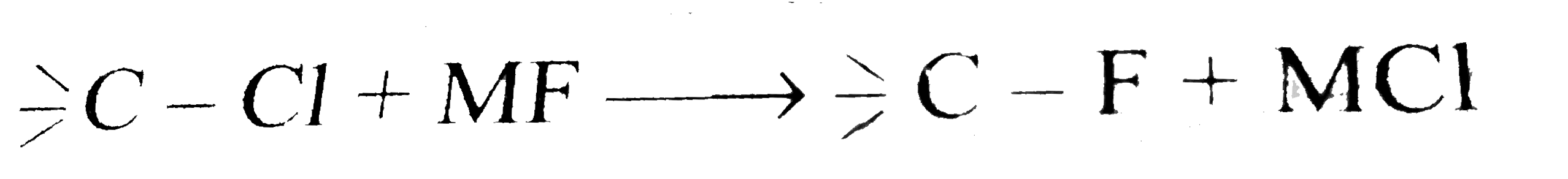
- Why does the reaction. proceed better with KF than with NaF ?
Text Solution
|
- why lithium is kept wrapped in paraffin wax and not stored in kerosene...
Text Solution
|
- When is a cation highly polarising? Which alkali metal cation has the ...
Text Solution
|
- Why cesium can be used in photoelectric cell, while lithium cannot be ...
Text Solution
|
- Give reason for the decreasing order of the conductivity of the follow...
Text Solution
|
- NaHCO(3) and NaOH cannot exist together in solution. Why ?
Text Solution
|
- On exposure to air, sodium hydroxide becomes liquid and after sometime...
Text Solution
|
- Alkali metals are obtained by the electrolysis of the molten salts and...
Text Solution
|
- What happens when: a. Potassium metal is dropped in water b. Potas...
Text Solution
|
- How is sodium carbonate manufactured by Solvay's process? Draw a schem...
Text Solution
|
- a. Describe one method of manufacture of castic soda. b. What happen...
Text Solution
|
- Answer the following: Give the name of the hardest alakli metal.
Text Solution
|
- Explain the following: a. Alkali metals are paramagnetic, but their ...
Text Solution
|
- Identify (A) and (B) in the following: KO(2)+S overset(Delta)rarr (A)...
Text Solution
|
- LiOH has been used by astronauts. Explain the use with the help of rea...
Text Solution
|
- Give the composition and action of backing powder.
Text Solution
|