`Na_(2)CO_(3)+2SO_(2)+H_(2)O rarr 2NaHSO_(4)+CO_(2)`
`2NaHSO_(3)+Na_(2)CO_(3)rarr 2Na_(2)S_(2)O_(3)`
`2Na_(2)S_(2)O_(3)+I_(2)overset(Delta)rarr Na_(2)S_(4)O_(6)+2NaI`
Oxidation states of sulphur:
a. `NaHSO_(3)=1+1+x-6=0, :. X=4`
b. `Na_(2)SO_(3)=2+x-6=0, :.x=4`
c. `Na_(2)S_(2)O_(3)` (Sodium thiosulphate)
Average oxidation numer.
`Na_(2)S_(2)O_(3)=2xx1+2x+3(-2)=0`
`:. x= +2`
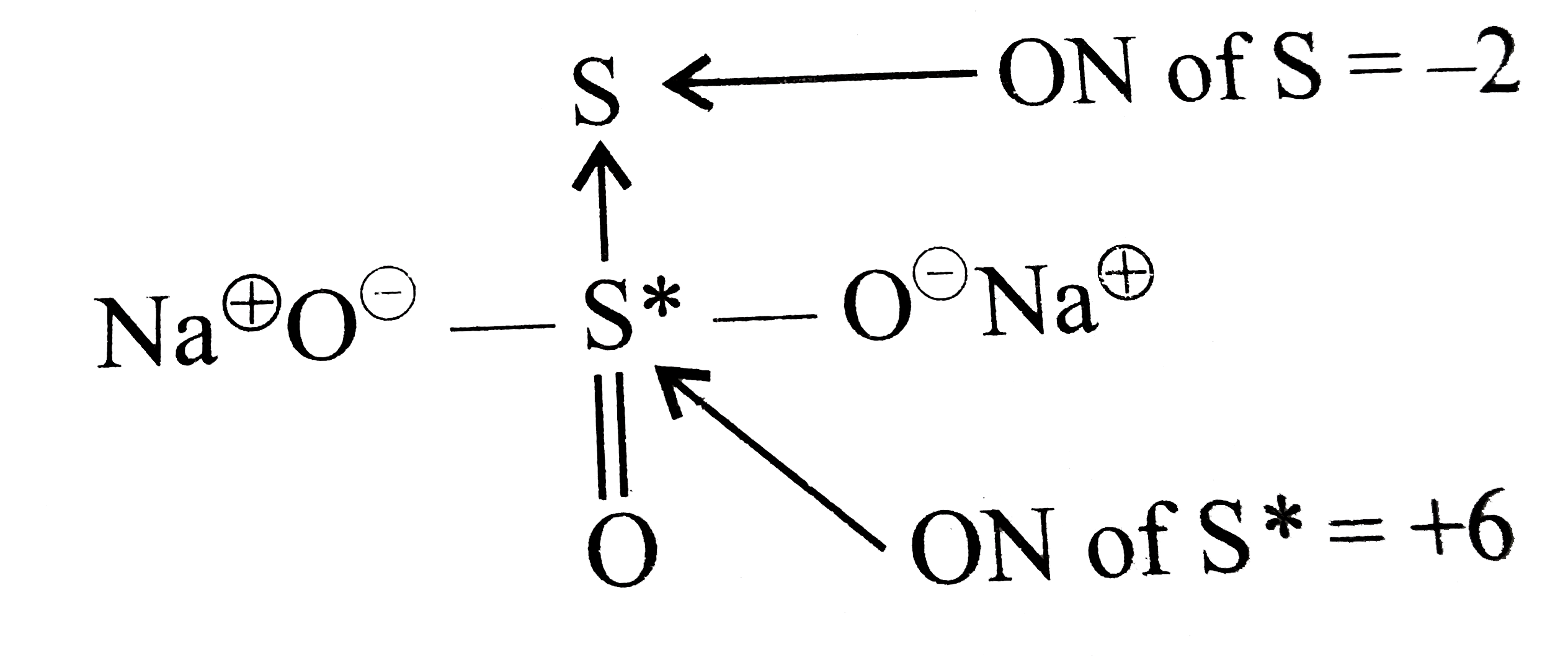
But oxidation number of `S= +2` is wrong because both the S atom cannot be in same oxidation state as is evident form the fact what `Na_(2)S_(2)O_(3)` is treated with dil `H_(2)SO_(4)`, one S atom gets precipitated while the other gets converted into `SO_(2)`. The oxidation numbers of these two S atom can, however, be determined by the chemical bonding method. By chemical bonding method, the structure of `Na_(2)S_(2)O_(3)` is `Na^(o+)O^(Θ)-overset(S)overset(uarr)underset(O)underset(||)S-O^(Θ)Na^(o+)`
Since there is a coordinate bond between two S atoms. Therefore, the acceptor S atom has an oxidation number of `-2`. The oxidation number of other S atoms can be calculated as follows:
`underset(("For Na"))(2xx(-1)) + underset(("For O atoms"))(3xx(-2)) + x + underset(("For coordinate S atom"))(1xx(-2)=0)`
OR
`+2-6+x-2=0, x= +6`
Thus, the two S atoms in `Na_(2)S_(2)O_(3)` have oxidation number of `-2` and `+6`.
d. Structure of tetrahionate ion is
`Na^(o+)O^(Θ)-overset(O)overset(||)underset(O)underset(||)(S^(+5))-S^(0)-S^(0)-overset(O)overset(||)underset(O)underset(||)(S^(+5))-O^(Θ)Na^(o+)`
Since each of the two terminal S atoms is connected to two oxygen atoms by a double bond and one oxygen atom by a single bond, therefore, the oxidation state of each of these terminal S atoms is `+5`. Since two central S atoms are linked to each other by a single bond, each S is further attached to similar species on either side, the electron pair formaing the `S-S` bond remains in the center and hence each of the two central S-atom has an oxidation state of zero. Therefore, oxidation number of two S-atoms are `(+5 and 0)`. However, the average oxidation state of the four S atoms is
2 S atoms in `+5` oxidation state and 2 S atoms in zero oxidation state.
`:. ((2xx5)+(2xx0))/(4)=2.5`
