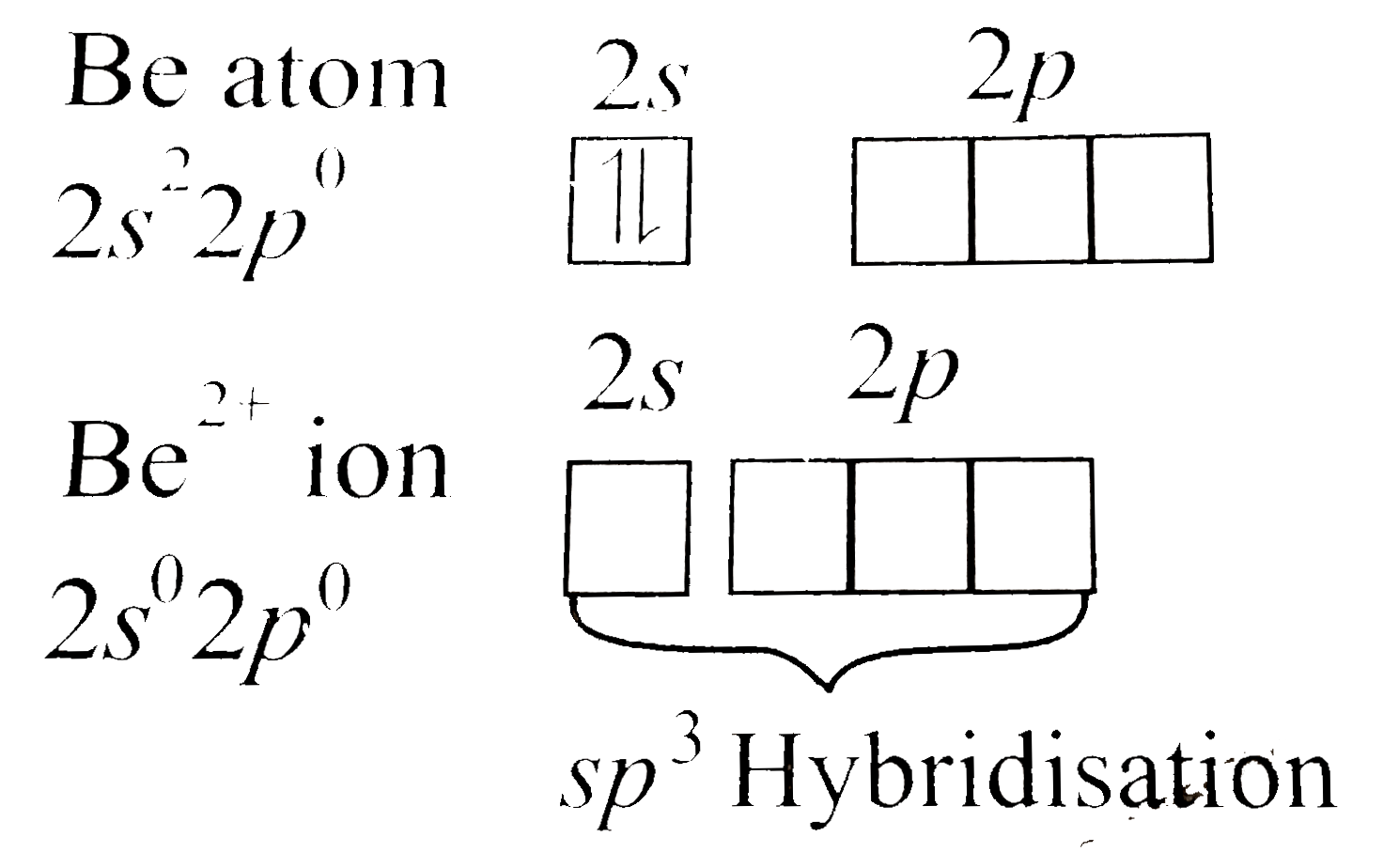
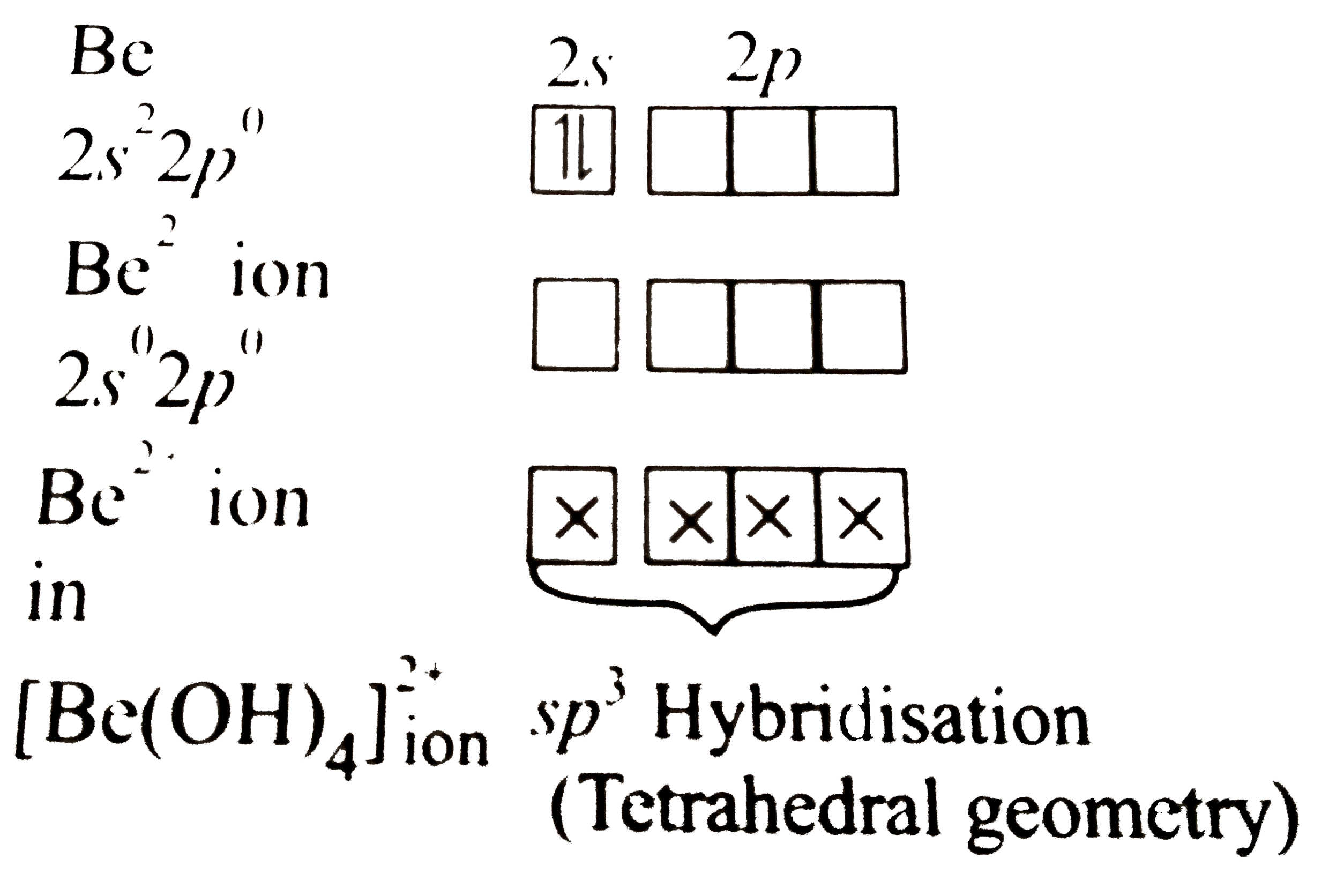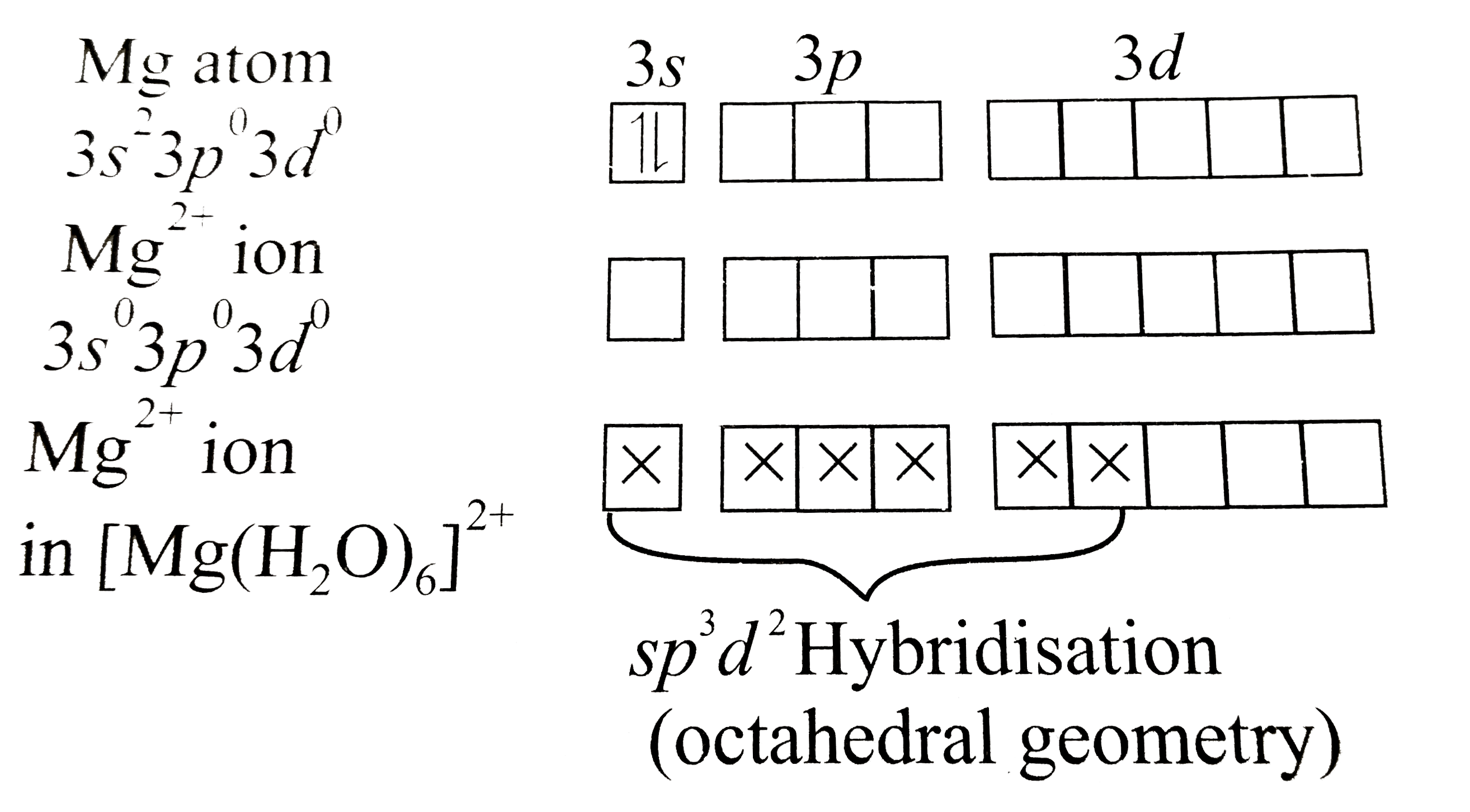Text Solution
Verified by Experts
Topper's Solved these Questions
S-BLOCK GROUP 2 - ALKALINE EARTH METALS
CENGAGE CHEMISTRY ENGLISH|Exercise Solved Example|6 VideosS-BLOCK GROUP 2 - ALKALINE EARTH METALS
CENGAGE CHEMISTRY ENGLISH|Exercise Exercises Linked Comprehension|42 VideosS-BLOCK GROUP 1 - ALKALI METALS
CENGAGE CHEMISTRY ENGLISH|Exercise Archives Subjective|8 VideosSOME BASIC CONCEPTS AND MOLE CONCEPT
CENGAGE CHEMISTRY ENGLISH|Exercise Archives Subjective|11 Videos
Similar Questions
Explore conceptually related problems