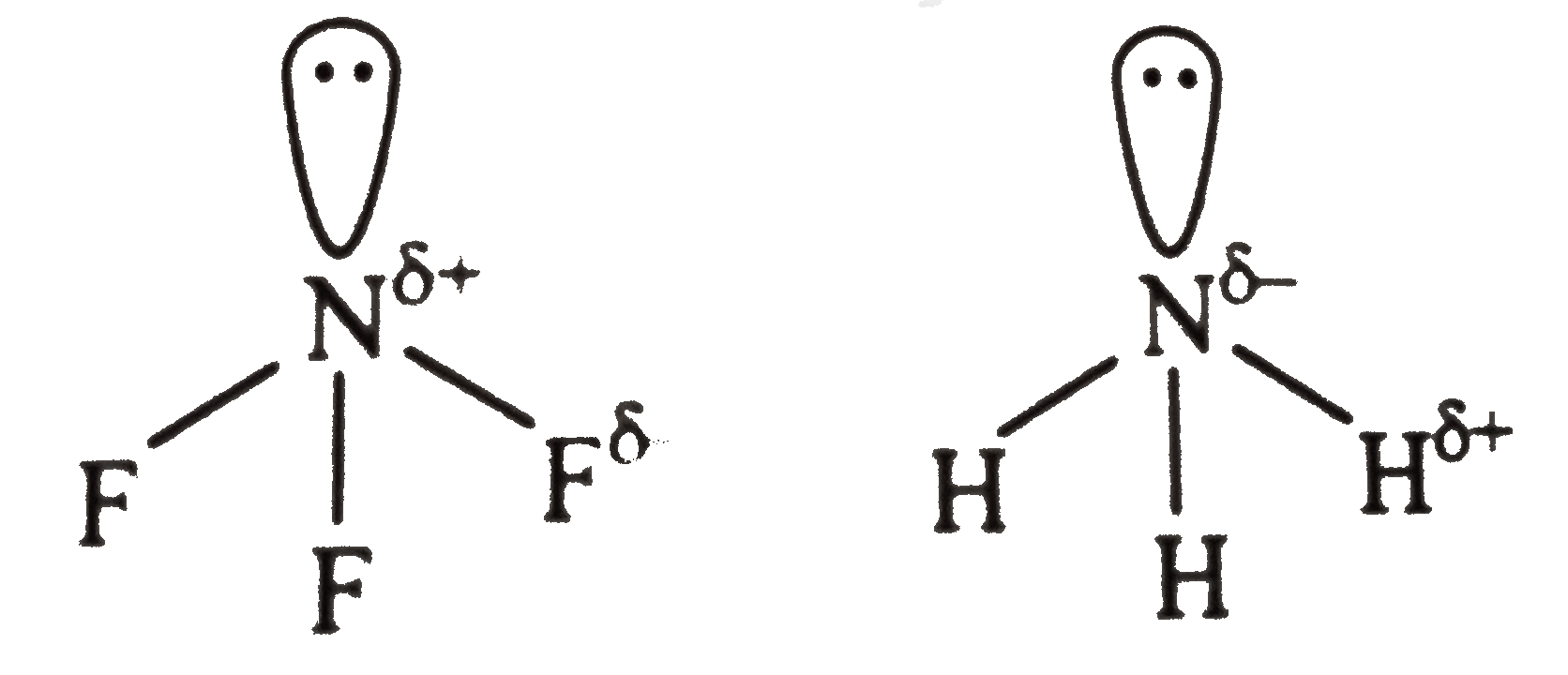Text Solution
Verified by Experts
Topper's Solved these Questions
P-BLOCK GROUP 15 ELEMENTS - THE NITROGEN FAMILY
CENGAGE CHEMISTRY ENGLISH|Exercise Concept Application Exercises 2.1 (Objective)|10 VideosP-BLOCK GROUP 15 ELEMENTS - THE NITROGEN FAMILY
CENGAGE CHEMISTRY ENGLISH|Exercise Exercises (Linked Comprehension)|22 VideosP-BLOCK GROUP 15 ELEMENTS - THE NITROGEN FAMILY
CENGAGE CHEMISTRY ENGLISH|Exercise Solved Example|7 VideosORGANIC COMPOUNDS WITH FUNCTIONAL GROUP
CENGAGE CHEMISTRY ENGLISH|Exercise Archives Analytical And Descriptive|24 VideosP-BLOCK GROUP 16 ELEMENTS - THE OXYGEN FAMILY
CENGAGE CHEMISTRY ENGLISH|Exercise Archives Subjective|10 Videos
Similar Questions
Explore conceptually related problems
CENGAGE CHEMISTRY ENGLISH-P-BLOCK GROUP 15 ELEMENTS - THE NITROGEN FAMILY-Concept Application Exercises 2.1 (Subjective)
- Why nitrogen is inert at room temperature ?
Text Solution
|
- Why is BiH(3) the strongest reducing agent amongst all the hydrides of...
Text Solution
|
- Why nitrogen trihalide cannot be oxidised to pentahalide whereas phosp...
Text Solution
|
- NO is paramagnetic, while NO^(oplus) is diamagnetic.
Text Solution
|
- Which of the following acts as reducing agent and why ? H3 PO3 or H3 P...
Text Solution
|
- Nitrogen is a gas, while other members of group 15 are solids. Why ?
Text Solution
|
- Commercial nitric acid is yellow in colour. Give reason.
Text Solution
|
- Nitric acid acts as an oxidising agent while nitrous acid can act both...
Text Solution
|
- Which is more basic and why ? NF3 or NH3.
Text Solution
|
- What is the chemistry of Holme's signal ?
Text Solution
|
- Write complete balanced reactions for the following : (a) Red phosph...
Text Solution
|
- Describe the action of heat on the following compounds : (a) Ammoniu...
Text Solution
|
- Explain why : Conc. nitric acid can be stored in aluminium containe...
Text Solution
|
- Why calcuim cyanamide can be used as a fertiliser ?
Text Solution
|
- Describe being odd electron molecule, NO is colourless. Explain.
Text Solution
|
- Give reason : Urea is better nitrogenous fertiliser than ammonium sulp...
Text Solution
|
- Mg3 N2 on reaction with water gives off NH3, but MgCl2 on reaction wit...
Text Solution
|
- Oxides of nitrogen have open chain structures while those of phosphoro...
Text Solution
|
- Illustrate how copper metal can give different products on reaction wi...
Text Solution
|
- Give the names of formulae of the compounds described below : (a) A ...
Text Solution
|