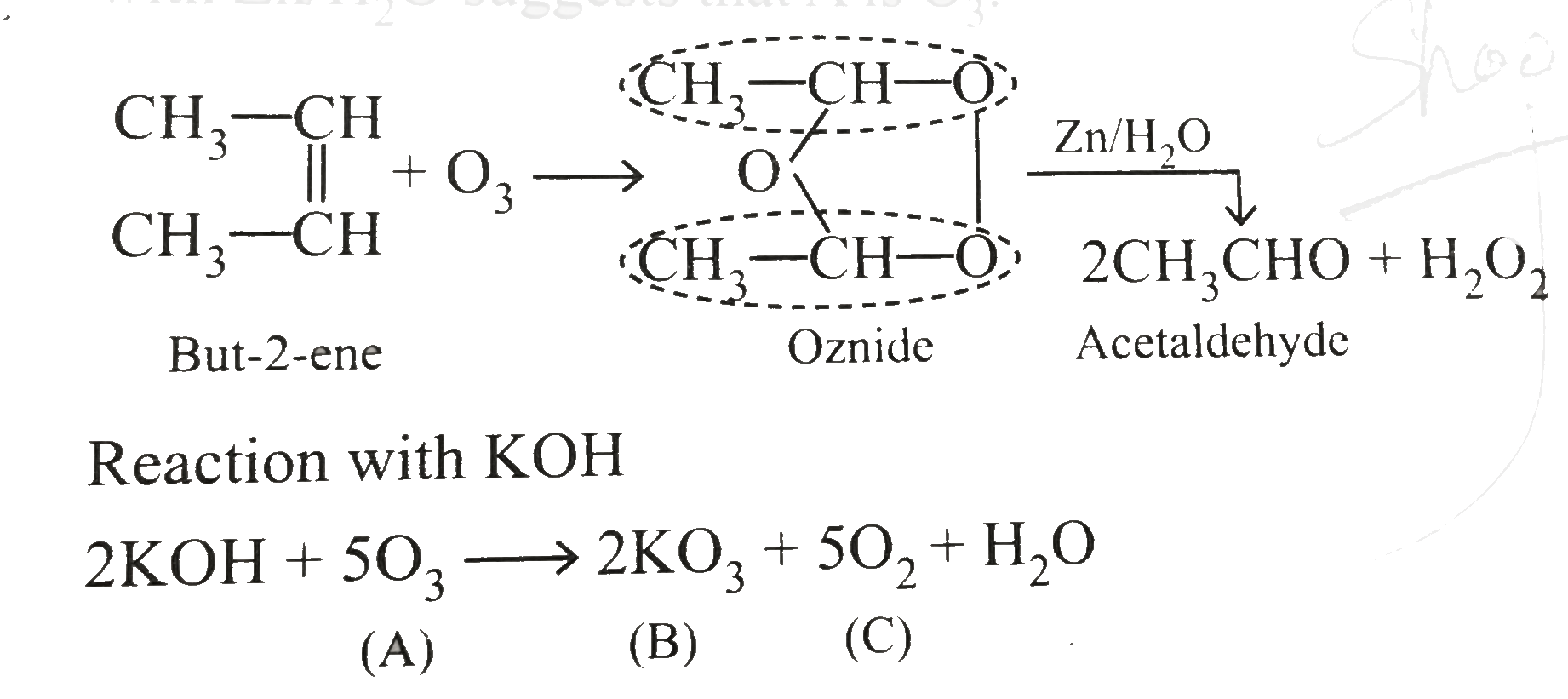
Text Solution
Verified by Experts
Topper's Solved these Questions
P-BLOCK GROUP 16 ELEMENTS - THE OXYGEN FAMILY
CENGAGE CHEMISTRY ENGLISH|Exercise Ex 3.1 Subjective (Give Reason :)|14 VideosP-BLOCK GROUP 16 ELEMENTS - THE OXYGEN FAMILY
CENGAGE CHEMISTRY ENGLISH|Exercise Ex 3.1Objective (Choose The Correct Option)|11 VideosP-BLOCK GROUP 16 ELEMENTS - THE OXYGEN FAMILY
CENGAGE CHEMISTRY ENGLISH|Exercise Archives Subjective|10 VideosP-BLOCK GROUP 15 ELEMENTS - THE NITROGEN FAMILY
CENGAGE CHEMISTRY ENGLISH|Exercise Exercises Archives (Subjective)|28 VideosP-BLOCK GROUP 17 ELEMENTS - THE HALOGEN FAMILY
CENGAGE CHEMISTRY ENGLISH|Exercise True/False (Subjective)|14 Videos
Similar Questions
Explore conceptually related problems
CENGAGE CHEMISTRY ENGLISH-P-BLOCK GROUP 16 ELEMENTS - THE OXYGEN FAMILY-Solved Example
- State with balanced equation what happens when ? (a) Potassium ferro...
Text Solution
|
- When gas (A) is passed through dry KOH at low temperature, a deep red ...
Text Solution
|
- (a) Sulphur melts form a clear mobile liquid at 119^@ C but on further...
Text Solution
|
- Concentrated H2 SO4 is added to the test tubes containing (a) to (e). ...
Text Solution
|
- A pale yellow substance (A) when heated with conc. HNO3 evolves a brow...
Text Solution
|