Text Solution
Verified by Experts
Topper's Solved these Questions
P-BLOCK GROUP 17 ELEMENTS - THE HALOGEN FAMILY
CENGAGE CHEMISTRY ENGLISH|Exercise Solved Example|7 VideosP-BLOCK GROUP 17 ELEMENTS - THE HALOGEN FAMILY
CENGAGE CHEMISTRY ENGLISH|Exercise Exs 4.1 (Subjective)|16 VideosP-BLOCK GROUP 16 ELEMENTS - THE OXYGEN FAMILY
CENGAGE CHEMISTRY ENGLISH|Exercise Archives Subjective|10 VideosP-BLOCK GROUP 18 ELEMENTS - THE INERT GASES
CENGAGE CHEMISTRY ENGLISH|Exercise Ex 5.1 (Objective)|14 Videos
Similar Questions
Explore conceptually related problems
CENGAGE CHEMISTRY ENGLISH-P-BLOCK GROUP 17 ELEMENTS - THE HALOGEN FAMILY-True/False (Subjective)
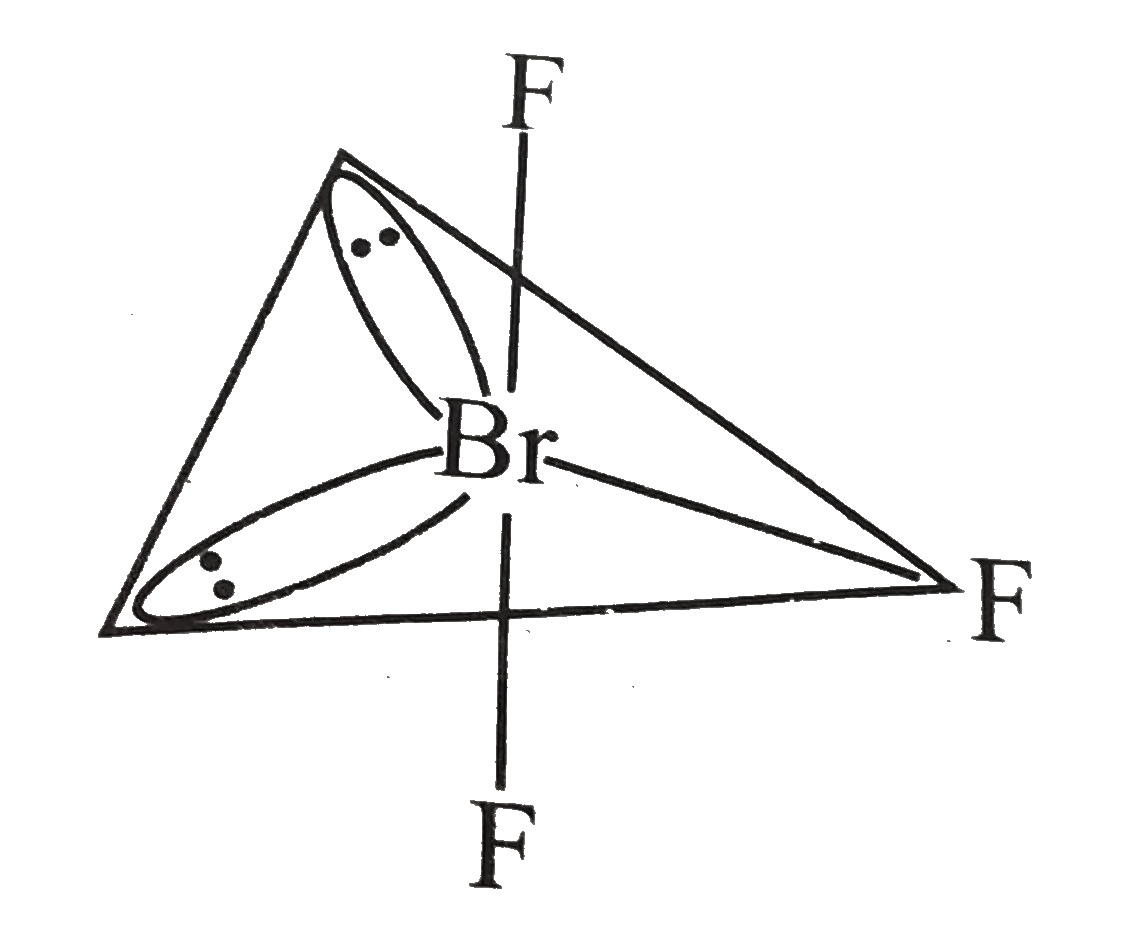
- Discuss the molecular shape of BrF(3) on the basis of VSEPR theory
Text Solution
|
- Give reason for the following within two sentences: i. Hydrogen bro...
Text Solution
|
- Complete and blance the following reactions: i. HNO(3)+HCI rarr NO...
Text Solution
|
- Show with balanced equations what happens when the following are mixed...
Text Solution
|
- Give reasons in one or two sentence for each of the following: Fluori...
Text Solution
|
- Write the blanced equations for the reactions when a mixture of potass...
Text Solution
|
- Arrange the following in the order of: i. Increase bond strength: ...
Text Solution
|
- Complete and balance the following reactions: CIO(3)^(Theta)+I^(Theta...
Text Solution
|
- Mention the producs formed in the following: ''Chlorine gas is bubble...
Text Solution
|
- Give balanced equations for the following: Iodate ion reacts with bis...
Text Solution
|
- Arrange the following: HOCI, HOCIO(2) and HOCIO in increazsing order ...
Text Solution
|
- Write the balanced chemical equations for the following: i. Sodium b...
Text Solution
|
- Account for the following : (i) NH(3) is a stronger base than PH(3) ...
Text Solution
|
- Complete the following chemical equations: KI+CI(2)rarr KCIO(2)+I(...
Text Solution
|
- Give an example of oxidation of halide by another halogen. Explain the...
Text Solution
|