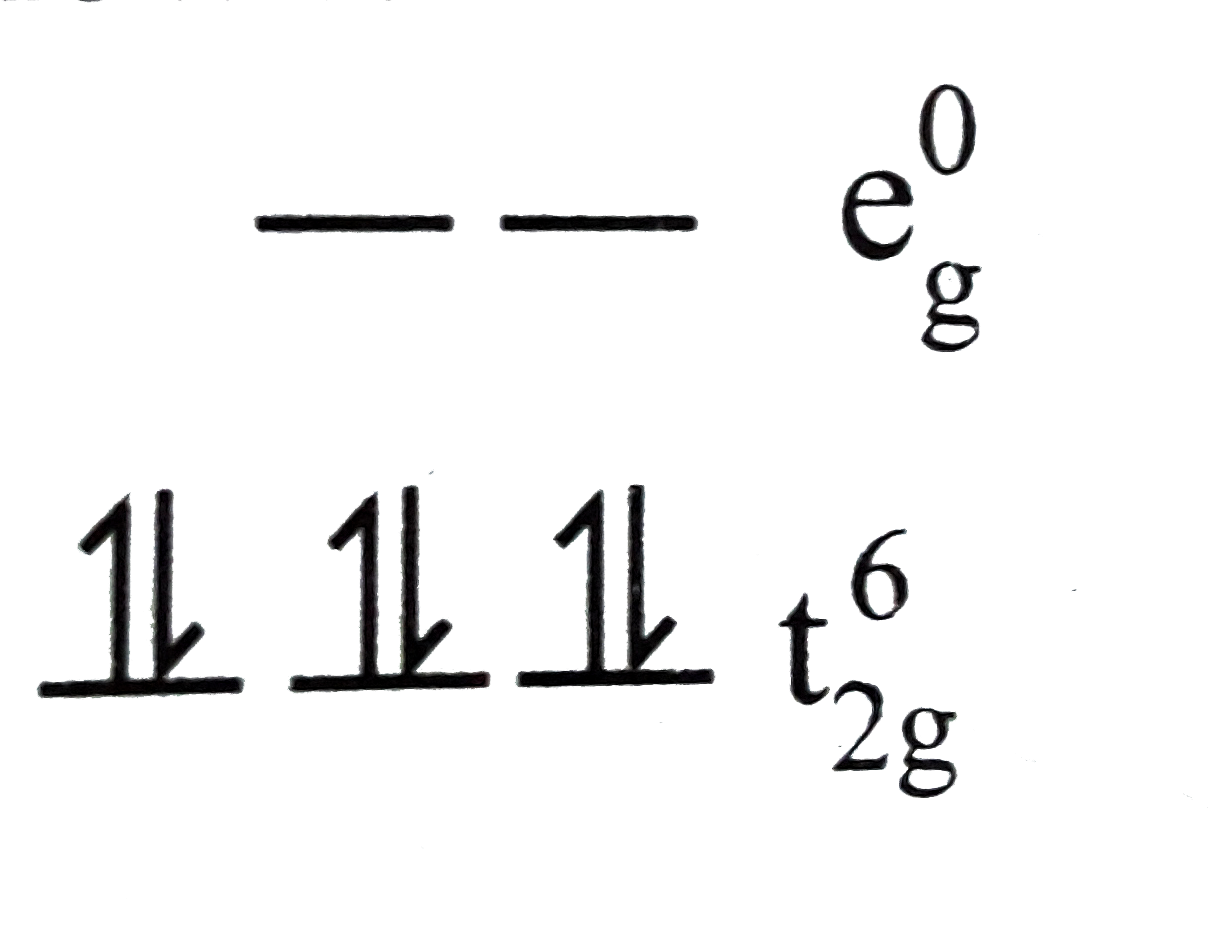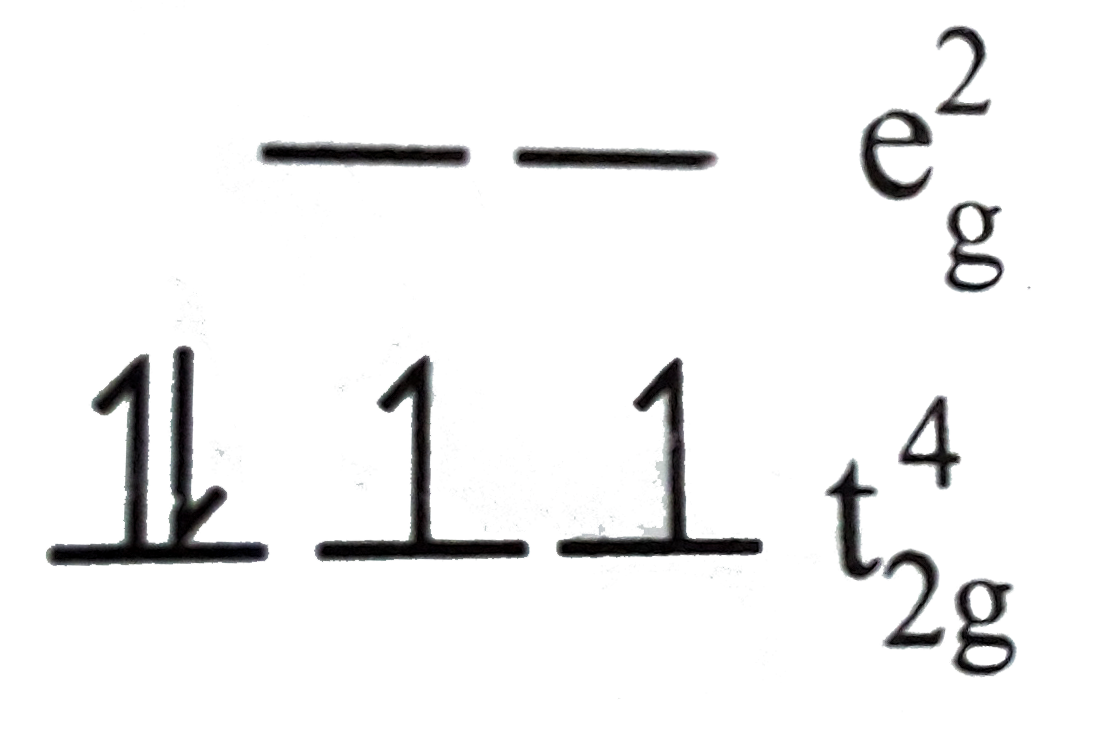Text Solution
Verified by Experts
Topper's Solved these Questions
COORDINATION COMPOUNDS
CENGAGE CHEMISTRY ENGLISH|Exercise Ex 7.2 Objective|8 VideosCOORDINATION COMPOUNDS
CENGAGE CHEMISTRY ENGLISH|Exercise Exercises Linked Comprehension|38 VideosCOORDINATION COMPOUNDS
CENGAGE CHEMISTRY ENGLISH|Exercise Ex 7.1 Objective (Isomerism)|12 VideosCHEMICAL KINETICS
CENGAGE CHEMISTRY ENGLISH|Exercise Archives Subjective|23 VideosD AND F BLOCK ELEMENTS
CENGAGE CHEMISTRY ENGLISH|Exercise Archives Subjective|29 Videos
Similar Questions
Explore conceptually related problems
CENGAGE CHEMISTRY ENGLISH-COORDINATION COMPOUNDS-Ex 7.2 Subjective
- One the basis of VBT answer the following complex ions [Ti(bpy)(3)]^...
Text Solution
|
- Identify the complex which are coloured and which are colourless Expla...
Text Solution
|
- Write the IUPAC nomenclature of the given complex along with its hybr...
Text Solution
|
- On the basic of CET explaine the following complex of Co^(3+) like [Co...
Text Solution
|