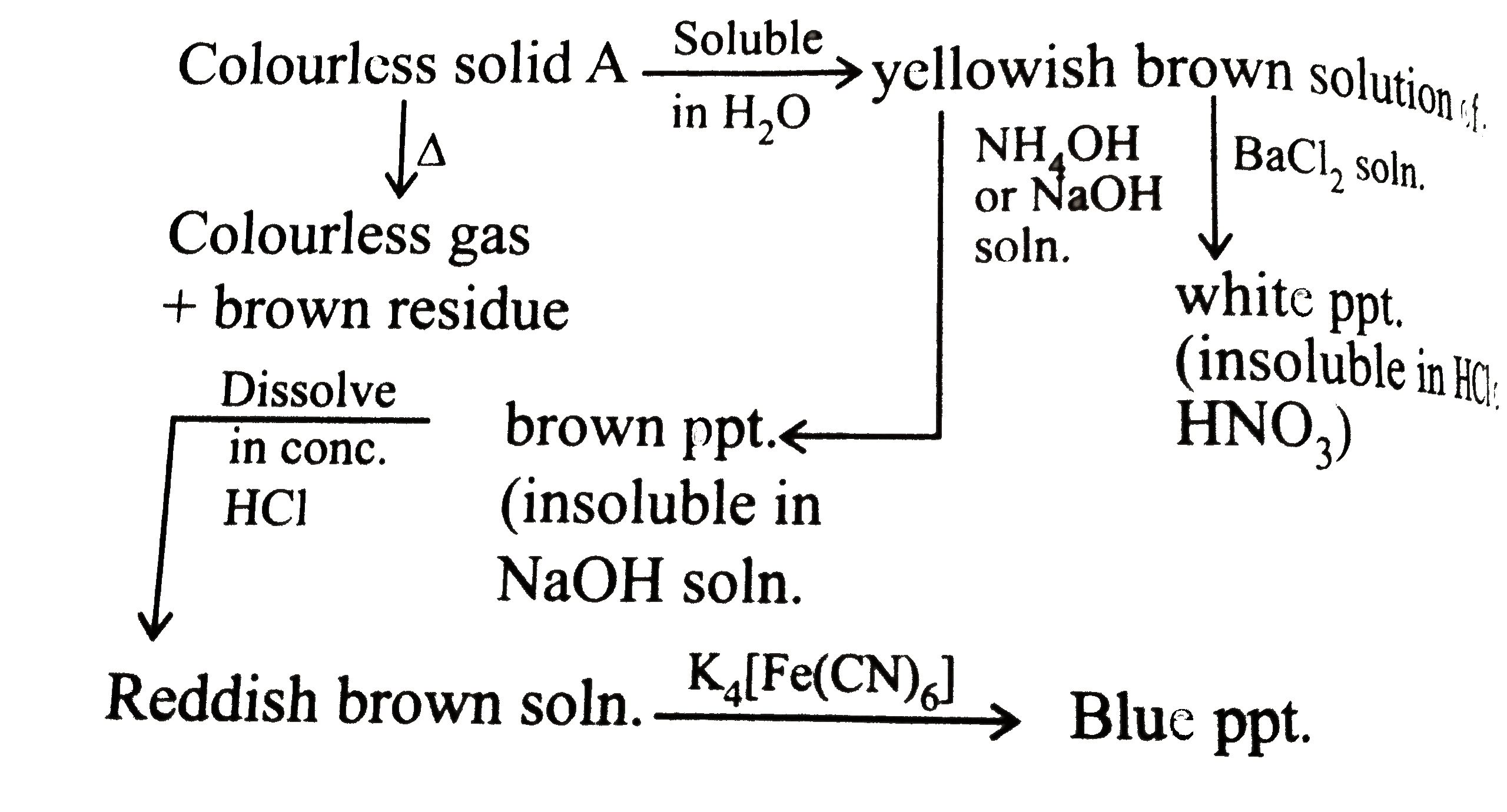
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
QUALITATIVE INORGANIC SALT ANALYSIS
CENGAGE CHEMISTRY ENGLISH|Exercise Exercises (Multiple Correct) Part-A (Analysis Of Anions)|30 VideosQUALITATIVE INORGANIC SALT ANALYSIS
CENGAGE CHEMISTRY ENGLISH|Exercise Exercises (Multiple Correct) Part-B (Analysis Of Cations)|28 VideosQUALITATIVE INORGANIC SALT ANALYSIS
CENGAGE CHEMISTRY ENGLISH|Exercise Solved Example|11 VideosP-BLOCK GROUP 18 ELEMENTS - THE INERT GASES
CENGAGE CHEMISTRY ENGLISH|Exercise Ex 5.1 (Objective)|14 VideosREDUCTION AND OXIDATION REACTION OF ORGANIC COMPOUNDS
CENGAGE CHEMISTRY ENGLISH|Exercise SUBJECTIVE TYPE|4 Videos
Similar Questions
Explore conceptually related problems
CENGAGE CHEMISTRY ENGLISH-QUALITATIVE INORGANIC SALT ANALYSIS-Exercises (Linked Comprehension)
- Identify A
Text Solution
|
- What is the formula of brown ppt?
Text Solution
|
- Which of the following complex is formed when A reacts with K(4)[Fe(...
Text Solution
|
- Identify A
Text Solution
|
- Identify B
Text Solution
|
- Give IUPAC name of K^(2)[Zn(OH)(4)]
Text Solution
|
- Identify D
Text Solution
|
- Identify A
Text Solution
|
- Identify B
Text Solution
|
- Identify C
Text Solution
|
- Complete the following chemical reactions. (i) PbS(s)+H 2 O 2 ...
Text Solution
|
- Identify A
Text Solution
|
- Identify B
Text Solution
|
- Identify C
Text Solution
|
- Identify A
Text Solution
|
- i.(A) overset(Delta)to glassy traparent beat (B) on platinum wire (...
Text Solution
|
- i.(A) overset(Delta)to glassy traparent beat (B) on platinum wire (...
Text Solution
|
- i.(A) overset(Delta)to glassy traparent beat (B) on platinum wire (...
Text Solution
|
- i.(A) overset(Delta)to glassy traparent beat (B) on platinum wire (...
Text Solution
|
- A colourless mixture of two salts (A) and (B) [excess] is soluble in H...
Text Solution
|