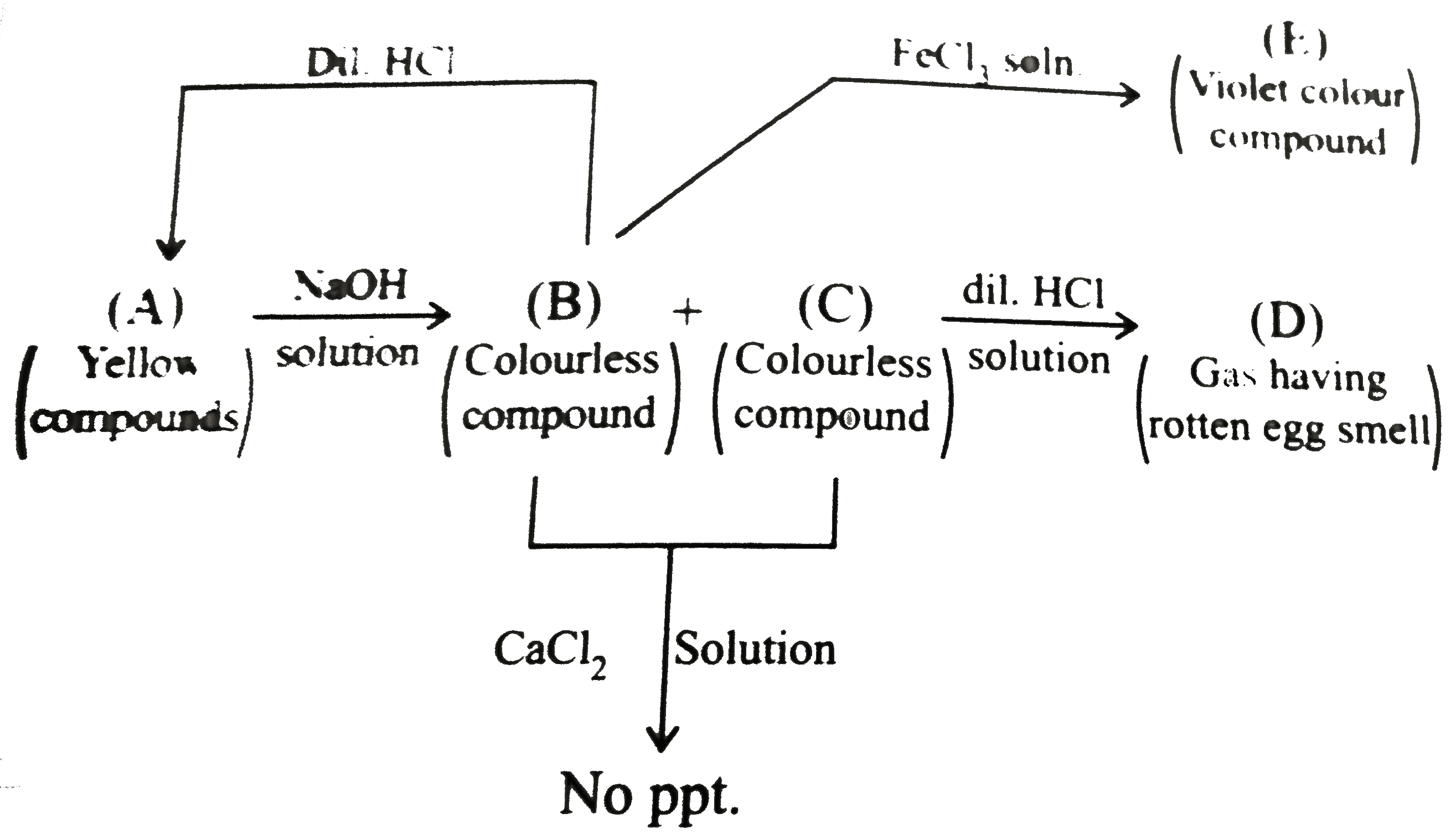
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
QUALITATIVE INORGANIC SALT ANALYSIS
CENGAGE CHEMISTRY ENGLISH|Exercise Exercises (Multiple Correct) Part-A (Analysis Of Anions)|30 VideosQUALITATIVE INORGANIC SALT ANALYSIS
CENGAGE CHEMISTRY ENGLISH|Exercise Exercises (Multiple Correct) Part-B (Analysis Of Cations)|28 VideosQUALITATIVE INORGANIC SALT ANALYSIS
CENGAGE CHEMISTRY ENGLISH|Exercise Solved Example|11 VideosP-BLOCK GROUP 18 ELEMENTS - THE INERT GASES
CENGAGE CHEMISTRY ENGLISH|Exercise Ex 5.1 (Objective)|14 VideosREDUCTION AND OXIDATION REACTION OF ORGANIC COMPOUNDS
CENGAGE CHEMISTRY ENGLISH|Exercise SUBJECTIVE TYPE|4 Videos
Similar Questions
Explore conceptually related problems
CENGAGE CHEMISTRY ENGLISH-QUALITATIVE INORGANIC SALT ANALYSIS-Exercises (Linked Comprehension)
- Find the anion (s)
Text Solution
|
- Find out (E )
Text Solution
|
- Find out (F)
Text Solution
|
- Cations are classified into varius group on the basis of their behav...
Text Solution
|
- Cations are classified into varius group on the basis of their behav...
Text Solution
|
- Cations are classified into varius group on the basis of their behav...
Text Solution
|
- The reagents like AgNO(3),K(4)[Fe(CN)(6)],KCNS,KI,K(2)CrO(4) Nessler's...
Text Solution
|
- The reagents like AgNO(3),K(4)[fe(CN)(6)],KCNS,KI,K(2)CrO(4) Nessler's...
Text Solution
|
- The reagents like AgNO(3),K(4)[Fe(CN)(6)],KCNS,KI,K(2)CrO(4) Nessler's...
Text Solution
|
- NH(3) solution was added to four semple solution in difference test t...
Text Solution
|
- NH(3) solution was added to four semple solution in difference test t...
Text Solution
|
- NH(3) solution was added to four semple solution in difference test t...
Text Solution
|
- (A) is a colourless solid, it metal when heated and gives of a gas (...
Text Solution
|
- (A) is a colourless solid, it metal when heated and gives of a gas (...
Text Solution
|
- (A) is a colourless solid, it metal when heated and gives of a gas (...
Text Solution
|
- Which of the following statement is/are correct for gas (D) ? (I)it h...
Text Solution
|
- Compound (B) on reaction with [Na(en)(3)][NO(3))(2) gives a coloured ...
Text Solution
|
- A colourless (A) when place into water a heavy white turbidly of (B) s...
Text Solution
|
- A colourless (A) when place into water a heavy white turbidly of (B) s...
Text Solution
|
- A colourless (A) when place into water a heavy white turbidly of (B) s...
Text Solution
|