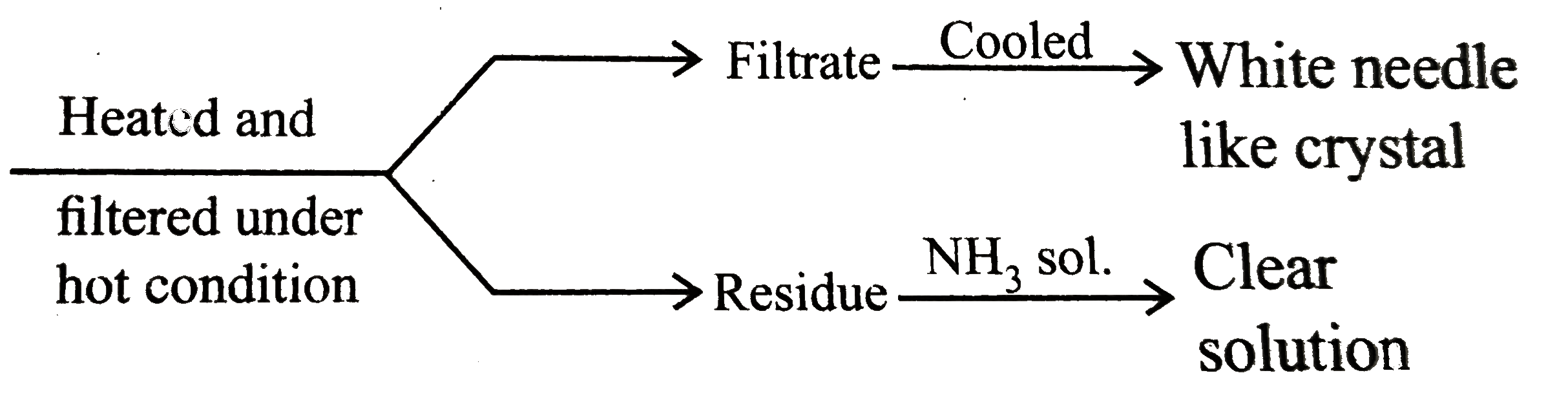
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
QUALITATIVE INORGANIC SALT ANALYSIS
CENGAGE CHEMISTRY ENGLISH|Exercise Exercises (Single Correct) Part-D (Miscellaneous)|6 VideosQUALITATIVE INORGANIC SALT ANALYSIS
CENGAGE CHEMISTRY ENGLISH|Exercise Exercises (Assertion-Reasion)|9 VideosQUALITATIVE INORGANIC SALT ANALYSIS
CENGAGE CHEMISTRY ENGLISH|Exercise Exercises (Single Correct) Part-B(Dry Test)|10 VideosP-BLOCK GROUP 18 ELEMENTS - THE INERT GASES
CENGAGE CHEMISTRY ENGLISH|Exercise Ex 5.1 (Objective)|14 VideosREDUCTION AND OXIDATION REACTION OF ORGANIC COMPOUNDS
CENGAGE CHEMISTRY ENGLISH|Exercise SUBJECTIVE TYPE|4 Videos
Similar Questions
Explore conceptually related problems
CENGAGE CHEMISTRY ENGLISH-QUALITATIVE INORGANIC SALT ANALYSIS-Exercises (Single Correct) Part-C (Analysis Of Cations)
- The presence of magnesium is confimed in the qualitative analysis by t...
Text Solution
|
- In qualitative inorganic analysis phosphate , if present, is to be eli...
Text Solution
|
- Na(2)CO(3) cannot be used in place of (NH(4))(2)CO(3) for the precipit...
Text Solution
|
- Disodium hydrogen phosphate is used to test :
Text Solution
|
- Reddish - brown (chocolate ) ppt. is formed with :
Text Solution
|
- Addition of SnCl(2) to HgCl(2) gives precipitate:
Text Solution
|
- To avoid the precipitation of hydroxides of Ni^(2+),Co^(2+),Zn^(2+) an...
Text Solution
|
- Which of the gives a white precipitate with a solution of AgNO(3), a ...
Text Solution
|
- In qualitative inorganic analysis of basic radicals hydrochloric acid ...
Text Solution
|
- Addition of solution of oxalate to an aqueous solution of mixture of B...
Text Solution
|
- The reagent that distinguishes between silver and lead salt is
Text Solution
|
- Sulphide ions react with Na(2)[Fe(NO)(CN)(5)] to form a purple coloure...
Text Solution
|
- The product of reaction of an aq solution of Bi^(3+) salt with sodium...
Text Solution
|
- Number of protons in Sodium and Sodium ion are same. True or False
Text Solution
|
- Few drop of HNO(3) are added to II group if before preceeding to group...
Text Solution
|
- A reddish pink substance on heating gives off a vapour which condenses...
Text Solution
|
- An inorganic Lewis acid (X) shows the following reactions : (a) It f...
Text Solution
|
- A colourless (X) is soluble in water and also in alcohol and amies...
Text Solution
|
- Salt mixture overset(dil. HCI) to Salt is consisting of cation
Text Solution
|
- Hg(NO(3))(2) overset(Delta) to W + X + O(2) X + H(2)O rarr HNO(2) + ...
Text Solution
|