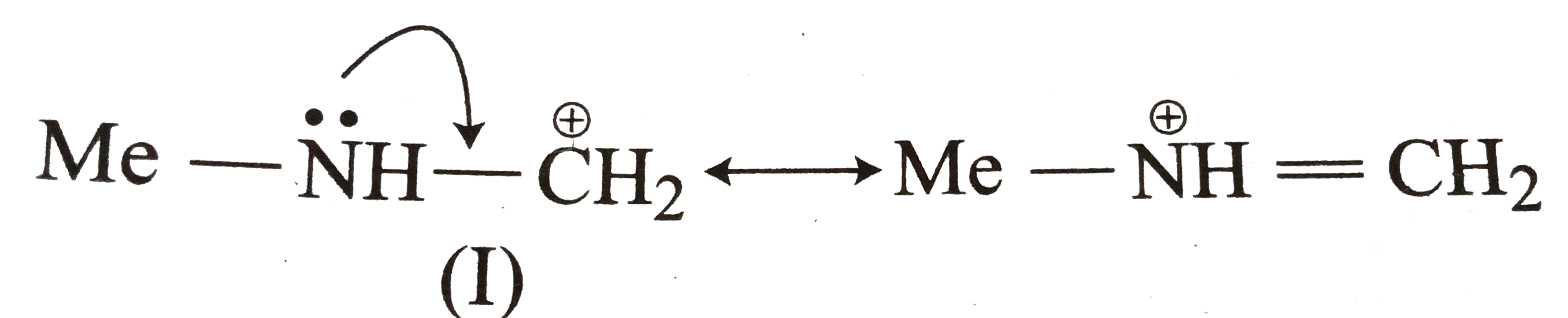Text Solution
Verified by Experts
Topper's Solved these Questions
GENERAL ORGANIC CHEMISTRY
CENGAGE CHEMISTRY ENGLISH|Exercise Subjective|11 VideosGENERAL ORGANIC CHEMISTRY
CENGAGE CHEMISTRY ENGLISH|Exercise Concept|17 VideosGENERAL ORGANIC CHEMISTRY
CENGAGE CHEMISTRY ENGLISH|Exercise Analytical and Descriptive|1 VideosCLASSIFICATION AND NOMENCLATURE OF ORGANIC COMPOUNDS
CENGAGE CHEMISTRY ENGLISH|Exercise Analytical and Descriptive Type|3 VideosHYDROGEN, WATER AND HYDROGEN PEROXIDE
CENGAGE CHEMISTRY ENGLISH|Exercise Subjective Archive (Subjective)|3 Videos
Similar Questions
Explore conceptually related problems
CENGAGE CHEMISTRY ENGLISH-GENERAL ORGANIC CHEMISTRY-Solved Example
- Which of the following is the most stable resonance structure .
Text Solution
|
- Which of the following is a most likely product from the reaction as s...
Text Solution
|
- Give the stability of the following resonance structures (a) H(2)C =...
Text Solution
|
- Write the correct resonance structure of the given compound. .
Text Solution
|
- The correct stability order for the following species is
Text Solution
|
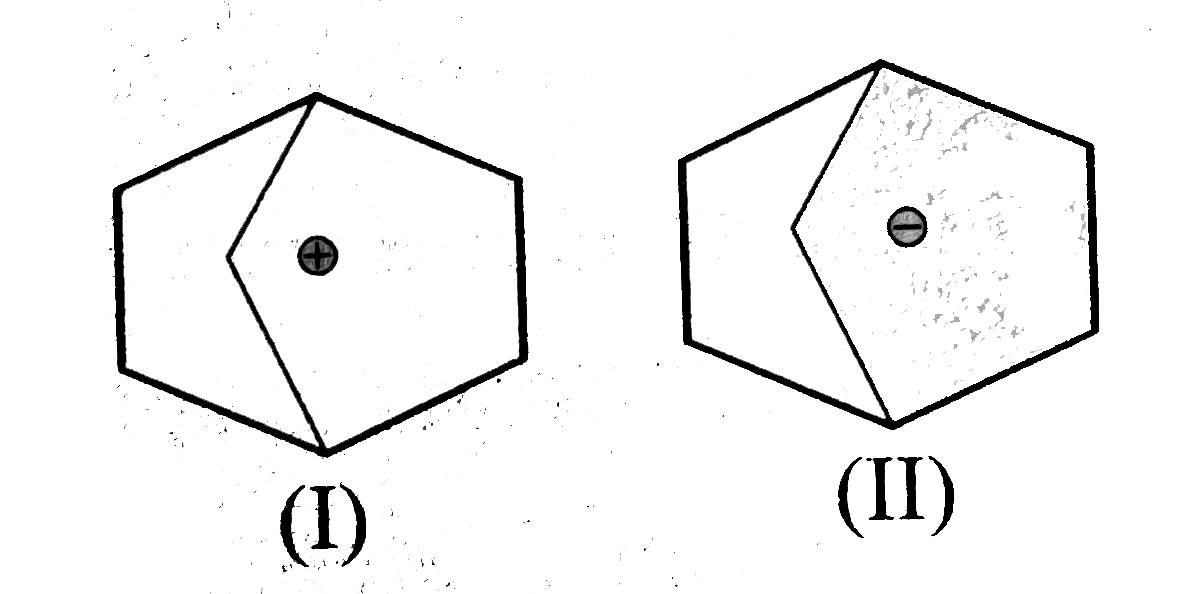
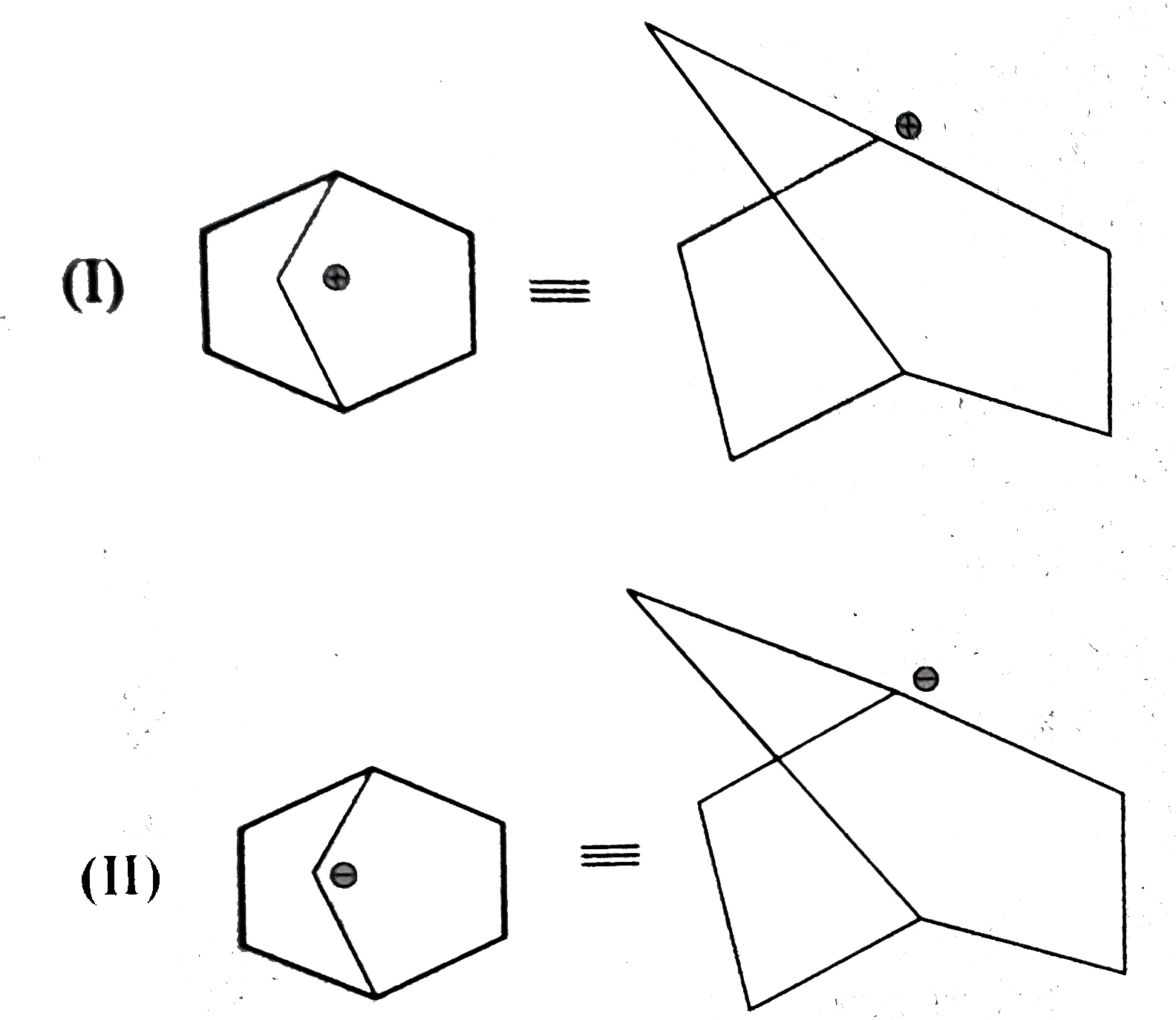
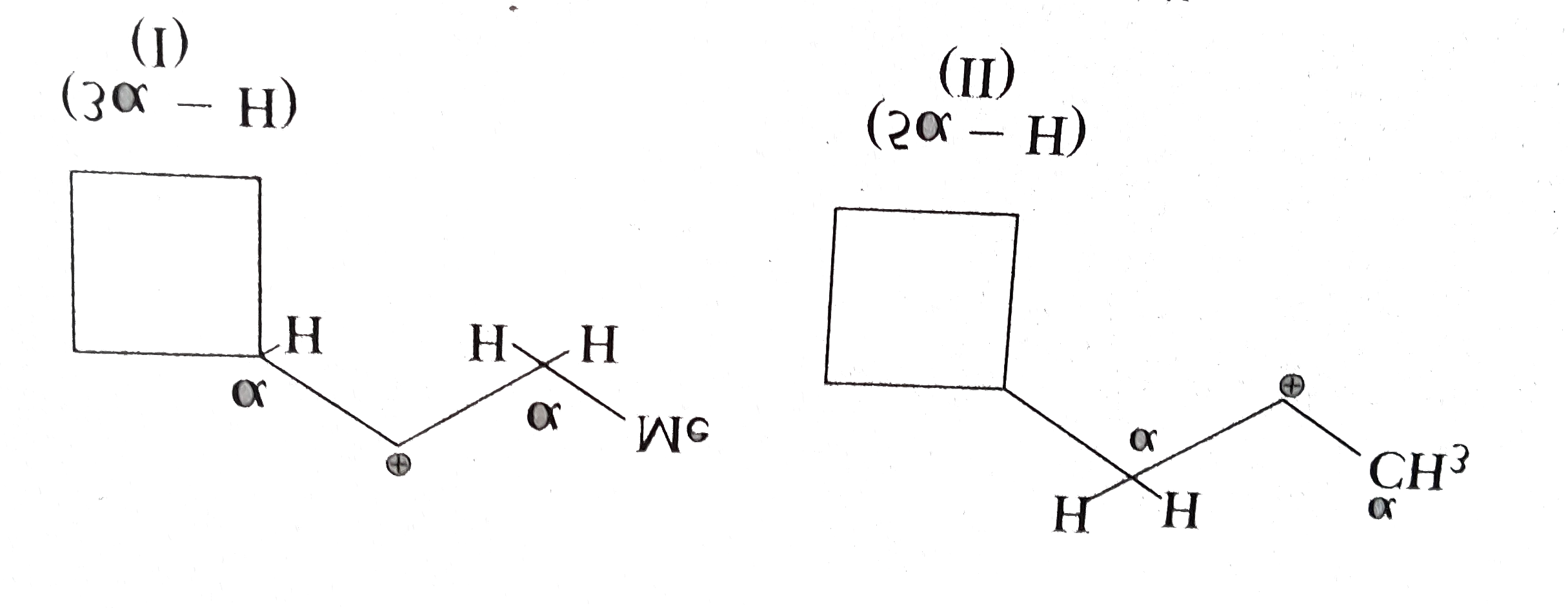
- Explain the following : (a) Why MeNH overset (oplus) CH2 (I) is more...
Text Solution
|
- Give decreasing order of the stabilities of the following :
Text Solution
|
- Arrange the following compounds in the order of increasing boiling poi...
Text Solution
|
- (I) Arrange the compounds (a0 in the order of decreasing boilling poin...
Text Solution
|
- Arrange the following in the decreasing order of boiling points : (i...
Text Solution
|
- Arrange the following alcohols : In the decreasing order of their bo...
Text Solution
|
- Arrange the following alcohols in the decreasing order of reactivity t...
Text Solution
|
- Arrange the following alchols in the decreasing order of their reactiv...
Text Solution
|
- Arrange the following in the decreasing order of acidity : (1) n-But...
Text Solution
|
- Arrange the following in the order of decreasing basic character : (1...
Text Solution
|
- Arrange in decreasing order of basicity. (1) m-BO2-C6H4-NH2 (2) C6...
Text Solution
|
- Compare the acidities of amide (R-underset(O)underset (||)(C)-NH(2)) a...
Text Solution
|
- Which of the following pairs would have higher boiling points ?
Text Solution
|