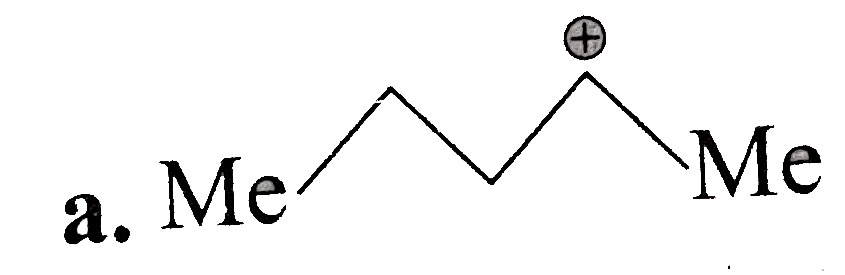
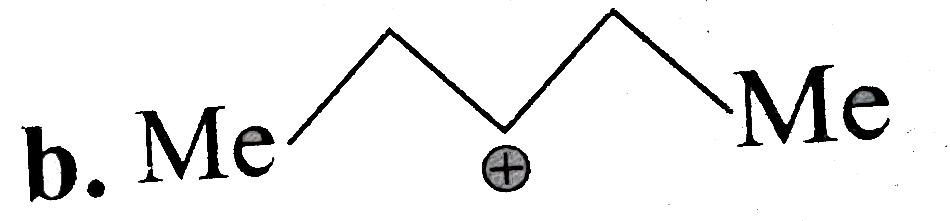
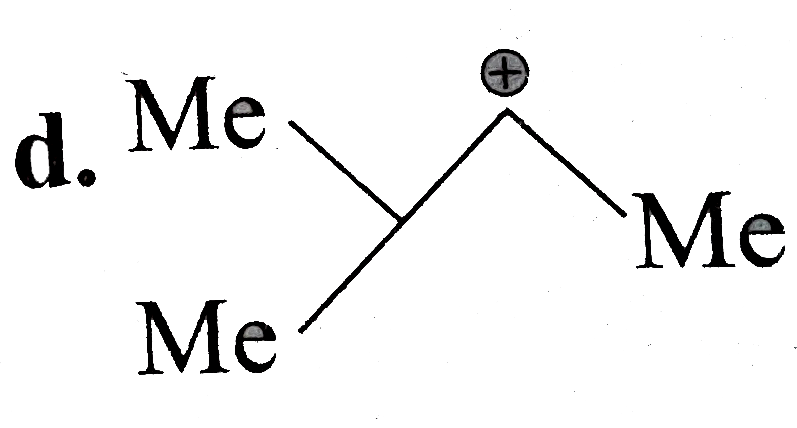
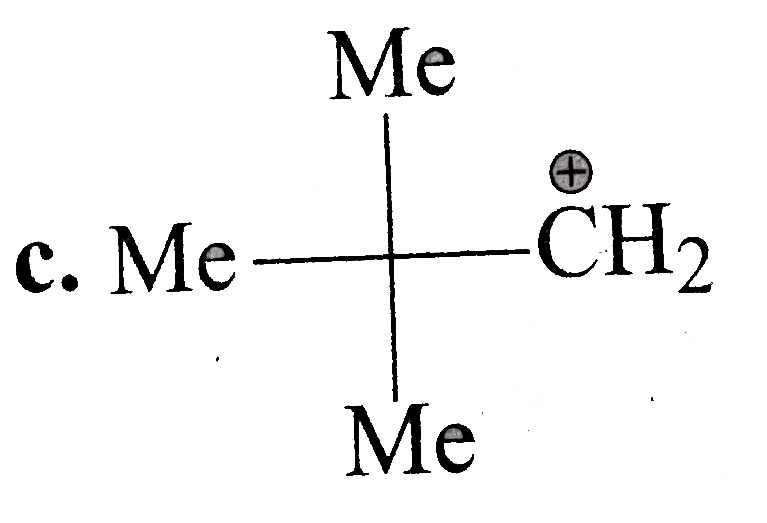A
B
C
D
Text Solution
AI Generated Solution
The correct Answer is:
Topper's Solved these Questions
GENERAL ORGANIC CHEMISTRY
CENGAGE CHEMISTRY ENGLISH|Exercise Assertion-Reasoning|5 VideosGENERAL ORGANIC CHEMISTRY
CENGAGE CHEMISTRY ENGLISH|Exercise Archives|12 VideosGENERAL ORGANIC CHEMISTRY
CENGAGE CHEMISTRY ENGLISH|Exercise Multiple Correct|22 VideosCLASSIFICATION AND NOMENCLATURE OF ORGANIC COMPOUNDS
CENGAGE CHEMISTRY ENGLISH|Exercise Analytical and Descriptive Type|3 VideosHYDROGEN, WATER AND HYDROGEN PEROXIDE
CENGAGE CHEMISTRY ENGLISH|Exercise Subjective Archive (Subjective)|3 Videos
Similar Questions
Explore conceptually related problems
CENGAGE CHEMISTRY ENGLISH-GENERAL ORGANIC CHEMISTRY-Single Correct
- Arrange the following in their decreasing order of Basicity. (I) ...
Text Solution
|
- Which of the following carbocations is least stable ?
Text Solution
|
- Which of the following carbocations is most stable ?
Text Solution
|
- The compound which gives the most stable carbonium ion on dehydration ...
Text Solution
|
- In the following graph, stability of different carbocations have been ...
Text Solution
|
- Which of the following is a soft base ?
Text Solution
|
- Which of the following is least stable ?
Text Solution
|
- Which of the following species is most stable ?
Text Solution
|
- The decreasing order of -I effect of the following is : (I) R3N^(opl...
Text Solution
|
- The decreasing order of-I effect of the following is : (I) CHO (II...
Text Solution
|
- The decreasing order of + I effect of the following is : (I) - O^(Ө)...
Text Solution
|
- The decreasing order of - I effect of the orbitals is : (I) sp (II...
Text Solution
|
- Give the decreasing order of hyperconjugative effect of R in R - CH = ...
Text Solution
|
- The decreasing order of the acidic character is : .
Text Solution
|
- The decreasing order of boiling points of the following is : (I) RCO...
Text Solution
|
- The decreasing order of basic character of the following is :
Text Solution
|
- The decreasing order of acidic character of the following is : .
Text Solution
|
- The decreasing order of acidic character of the following is : (I) p...
Text Solution
|
- The decreasing order of basic characters of the following is : (I) A...
Text Solution
|
- The increasing order of pKb value of the following is : (I) HC -= C^...
Text Solution
|