A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
GENERAL ORGANIC CHEMISTRY
CENGAGE CHEMISTRY ENGLISH|Exercise Fill in the blanks|4 VideosGENERAL ORGANIC CHEMISTRY
CENGAGE CHEMISTRY ENGLISH|Exercise Analytical and Descriptive|1 VideosGENERAL ORGANIC CHEMISTRY
CENGAGE CHEMISTRY ENGLISH|Exercise Assertion-Reasoning|5 VideosCLASSIFICATION AND NOMENCLATURE OF ORGANIC COMPOUNDS
CENGAGE CHEMISTRY ENGLISH|Exercise Analytical and Descriptive Type|3 VideosHYDROGEN, WATER AND HYDROGEN PEROXIDE
CENGAGE CHEMISTRY ENGLISH|Exercise Subjective Archive (Subjective)|3 Videos
Similar Questions
Explore conceptually related problems
CENGAGE CHEMISTRY ENGLISH-GENERAL ORGANIC CHEMISTRY-Archives
- Polarization in acrolein as:
Text Solution
|
- The compound which gives the most stable carbonium ion on dehydration ...
Text Solution
|
- Which of the following hydrocarbons has the lowest dipole moment.
Text Solution
|
- Which of the following molecules has highest dipole moment?
Text Solution
|
- Arrange in the order of increasing acidic strengths.
Text Solution
|
- Which of the following resonating structures of 1-methoxy-1,3-butadien...
Text Solution
|
- Which of the following is the most stable resonance structure .
Text Solution
|
- Hyperconjugation involves overlap of which of the following orbitals?
Text Solution
|
- The correct stability order for the following species is
Text Solution
|
- In the following carbocation, H//CH3 that is most likely to migrate to...
Text Solution
|
- The correct of acidities of the following is :
Text Solution
|
- Give the stability of the following resonance structures (a) H(2)C =...
Text Solution
|
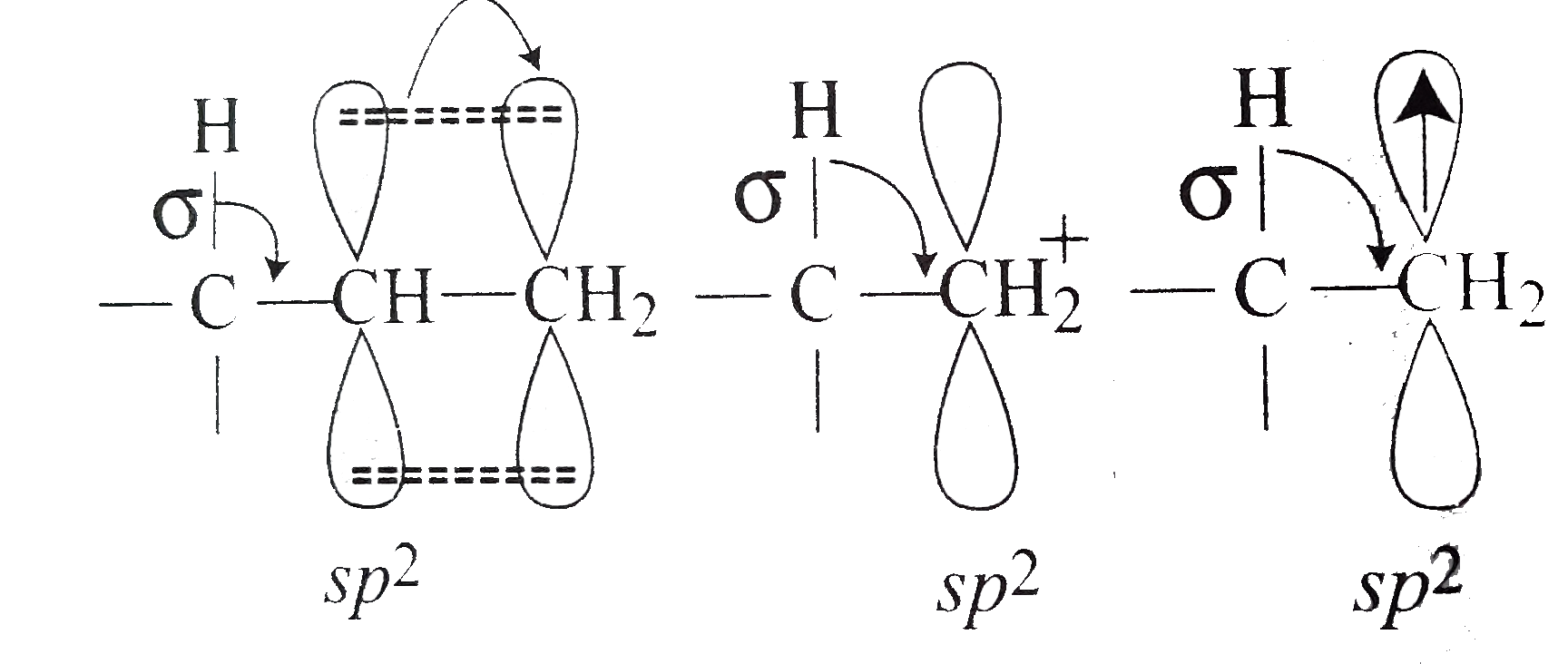 .
.