Text Solution
Verified by Experts
Topper's Solved these Questions
ORGANIC REACTION MECHANISM
CENGAGE CHEMISTRY ENGLISH|Exercise Subjective|19 VideosORGANIC REACTION MECHANISM
CENGAGE CHEMISTRY ENGLISH|Exercise Comprehension|35 VideosORGANIC REACTION MECHANISM
CENGAGE CHEMISTRY ENGLISH|Exercise Analytical and Descriptive|6 VideosNCERT BASED EXERCISE
CENGAGE CHEMISTRY ENGLISH|Exercise Chemical Equilibrium|73 VideosP-BLOCK GROUP 13 - BORON FAMILY
CENGAGE CHEMISTRY ENGLISH|Exercise Exercise Archives (Subjecive)|9 Videos
Similar Questions
Explore conceptually related problems
CENGAGE CHEMISTRY ENGLISH-ORGANIC REACTION MECHANISM-Solved Example
- Give the order of (a) nucleophilicity (b) basic character, and (c) fug...
Text Solution
|
- Give the most reactive substrate in each of the following pair with ov...
Text Solution
|
- Outline the synthesis of following compounds from suitable nucleophile...
Text Solution
|
- Give a suitable mechanism for the following : .
Text Solution
|
- Predict the order of reactivity of the following halides with (a) NaI ...
Text Solution
|
- Explain : (a)ClCH2OCH3 (chloromethyl methyl ether) undergoes SN^1 re...
Text Solution
|
- Write the products of the following SN reactions. .
Text Solution
|
- Complete the following reaction
Text Solution
|
- Bromobenzene can be dehydrobrominated to benzyne by three possible rou...
Text Solution
|
- Classify the following reactions as addition, elimination, substitutio...
Text Solution
|
- From the following elimination reactions, predict which one is Hofman...
Text Solution
|
- Which one is more reactive towards acidcatalysed hydration ? Predict t...
Text Solution
|
- Which one hydrolyses at a faster rate by SN^1 mechanism ? .
Text Solution
|
- Explain :
Text Solution
|
- What are the products of addition of D2 to trans-2-pentene.
Text Solution
|
- Meso-2,3-dibromobutane reacts with iodide ion more rapidly than does (...
Text Solution
|
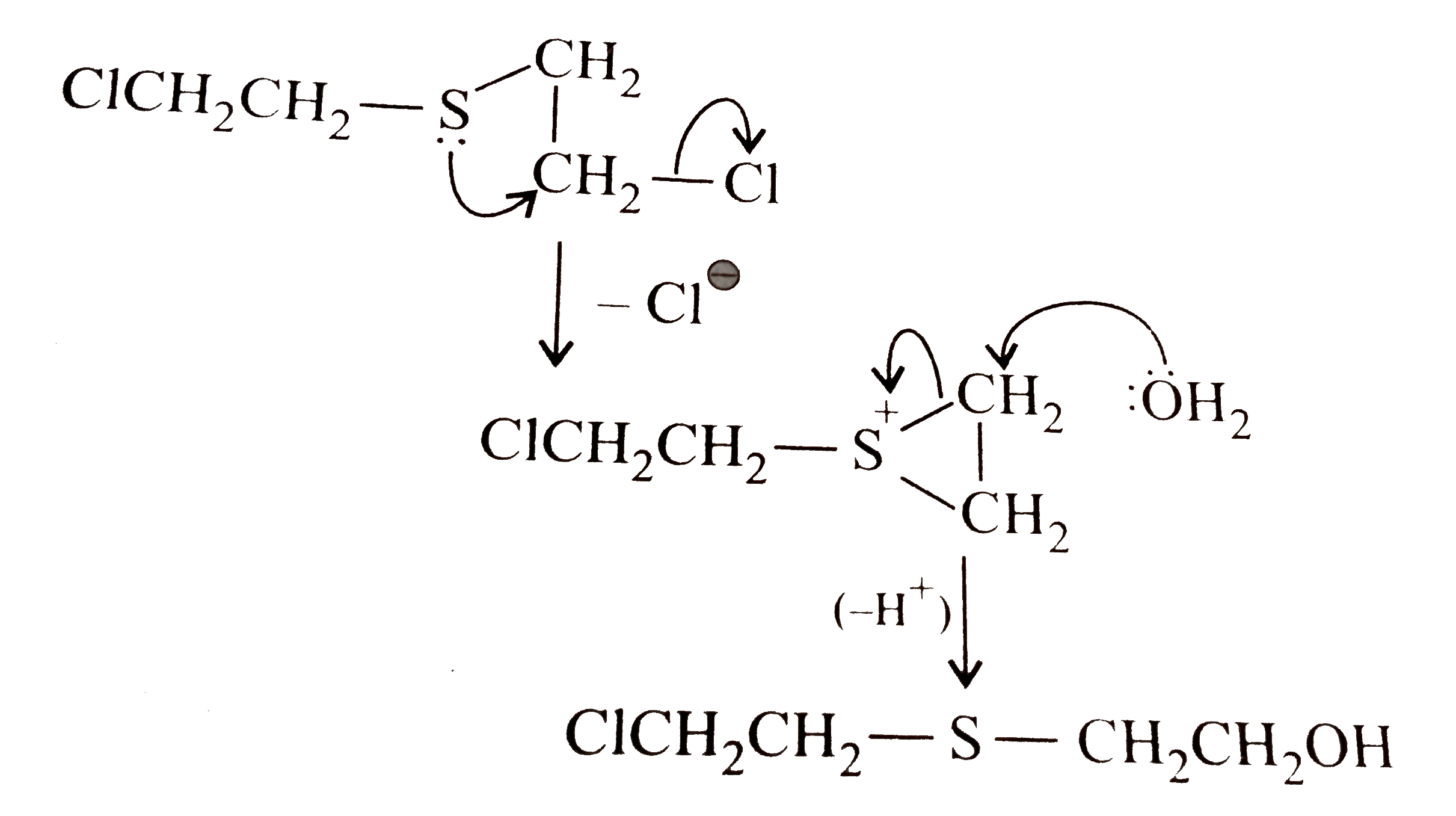
- Mustard gas (ClCH2CH2)2-S hydrolysed by water to Cl(CH2)2 S(CH2)2 OH m...
Text Solution
|
- Give the product, indicating stereo-chemistry of cyclohexene with Br2
Text Solution
|
- 1,2-Dimethylcyclohexene undergoes only trans-addition with HBr in no...
Text Solution
|
- Complete the following and state the relations between configurations ...
Text Solution
|