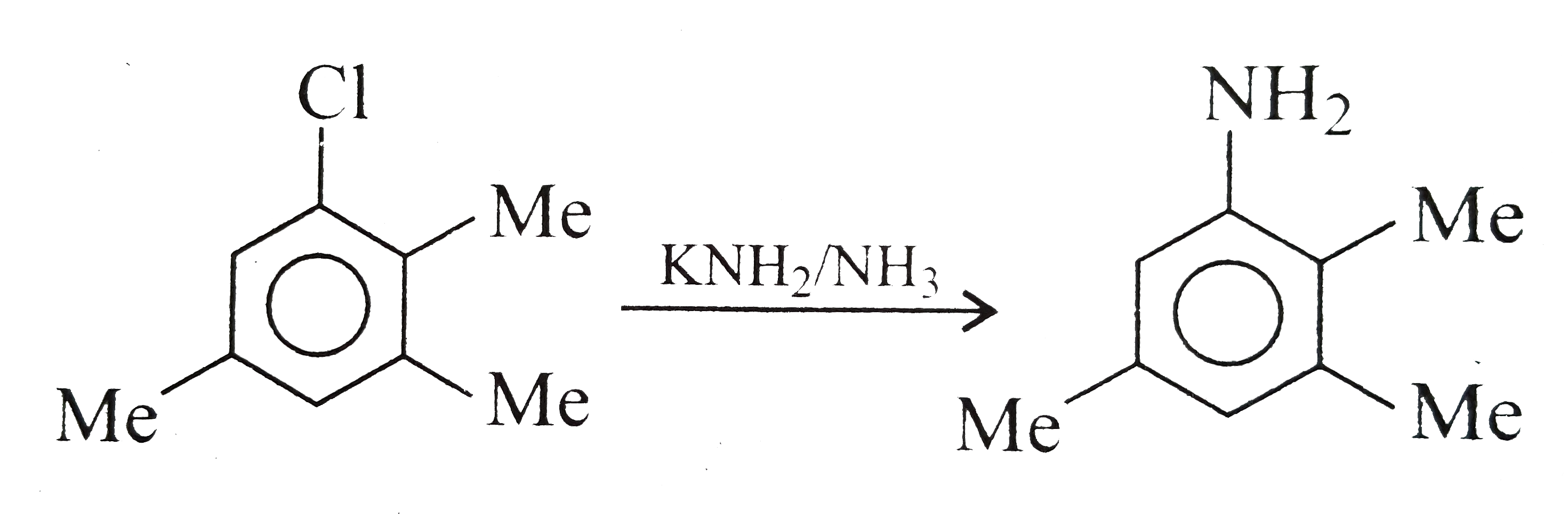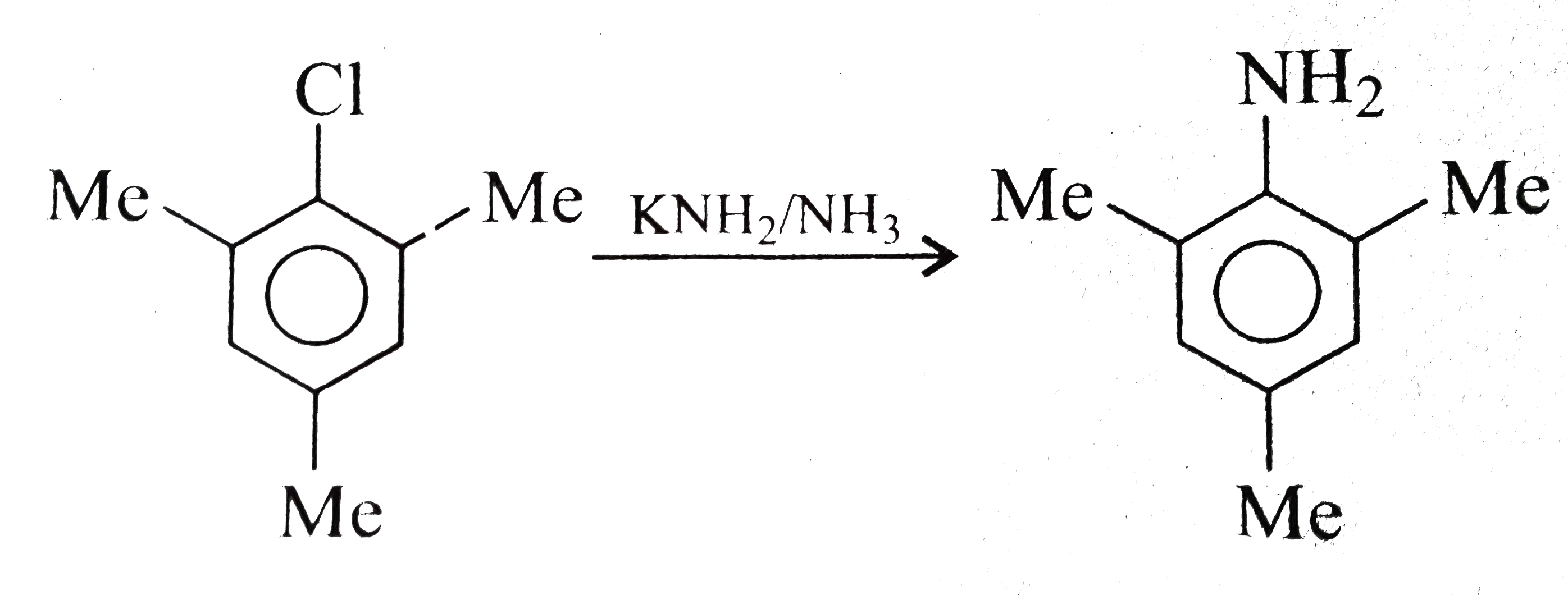A
B
C
D
Text Solution
AI Generated Solution
The correct Answer is:
Topper's Solved these Questions
ORGANIC REACTION MECHANISM
CENGAGE CHEMISTRY ENGLISH|Exercise Single correct|43 VideosORGANIC REACTION MECHANISM
CENGAGE CHEMISTRY ENGLISH|Exercise Assertion-Reasoning|12 VideosORGANIC REACTION MECHANISM
CENGAGE CHEMISTRY ENGLISH|Exercise Comprehension|35 VideosNCERT BASED EXERCISE
CENGAGE CHEMISTRY ENGLISH|Exercise Chemical Equilibrium|73 VideosP-BLOCK GROUP 13 - BORON FAMILY
CENGAGE CHEMISTRY ENGLISH|Exercise Exercise Archives (Subjecive)|9 Videos
Similar Questions
Explore conceptually related problems
CENGAGE CHEMISTRY ENGLISH-ORGANIC REACTION MECHANISM-Multiple Correct
- Which of the following statements are correct ?
Text Solution
|
- Which of the following reactions represents SN reaction ?
Text Solution
|
- Which of the following reactions are feasible ?
Text Solution
|
- Which of the following transformations are feasible ?
Text Solution
|
- In which of the following reactions, the intermediate species acyl nit...
Text Solution
|
- Which of the following statements are correct ?
Text Solution
|
- Which of the following is//are the rate determining step (s) of the gi...
Text Solution
|
- The products in the given reaction are : .
Text Solution
|
- Which of the following statements are correct about the addition of HB...
Text Solution
|
- Which of the following statements are correct ?
Text Solution
|
- In which of the following reactions, rearrangement is possible ?
Text Solution
|
- Which of the following is an example of nucleophilic addition to aceta...
Text Solution
|
- In which of the reactions, there is a migration of alkyl group to carb...
Text Solution
|
- Which of the following statements are correct ?
Text Solution
|
- Which of the following statements/reactions are correct ?
Text Solution
|
- Which of the following statements is//are correct ?
Text Solution
|
- Consider the following reactions : (I) Me - underset ((A)) -= - Me o...
Text Solution
|
- Consider the following reactions : Which of the following stateme...
Text Solution
|
- Consider the following reactions : Which of the following stateme...
Text Solution
|
- Which of the following statements/reactions are correct ?
Text Solution
|