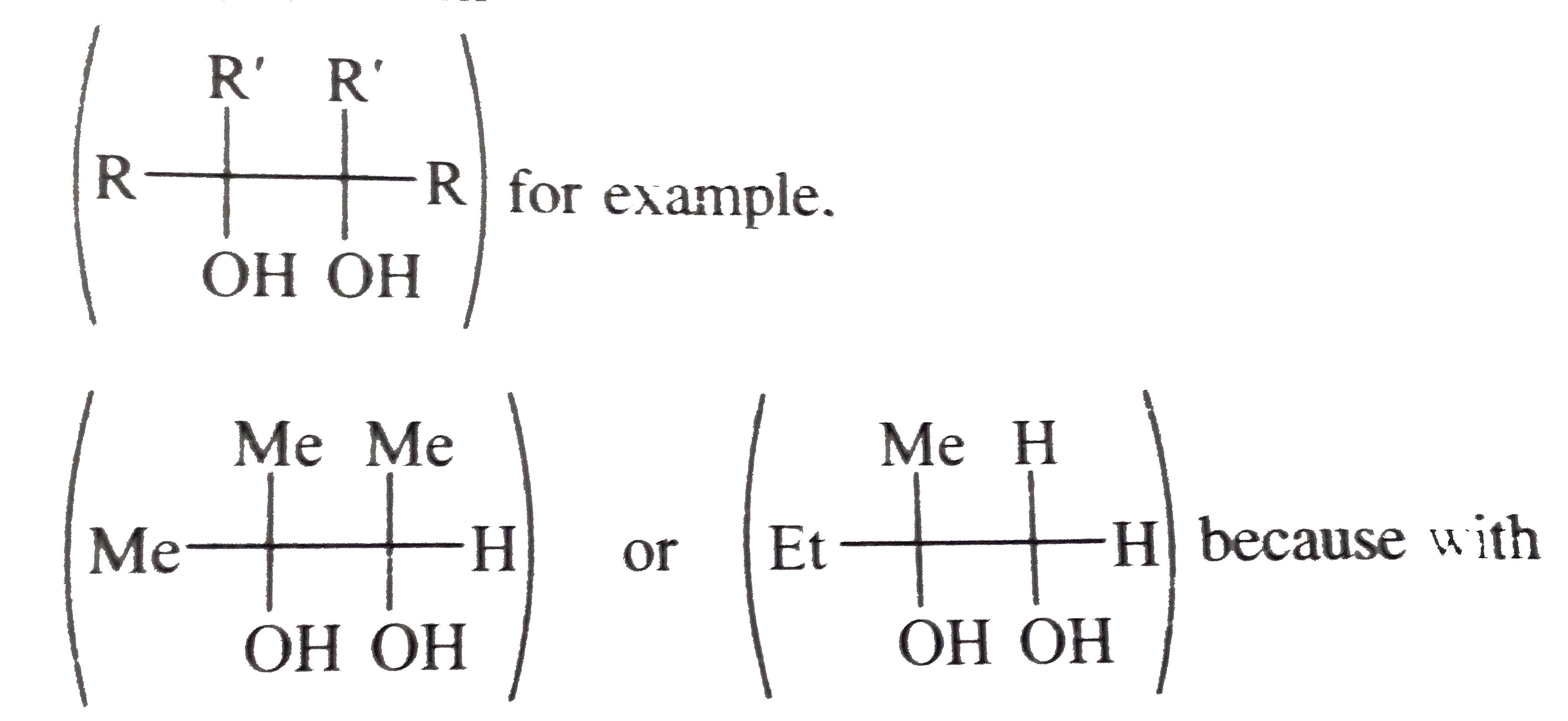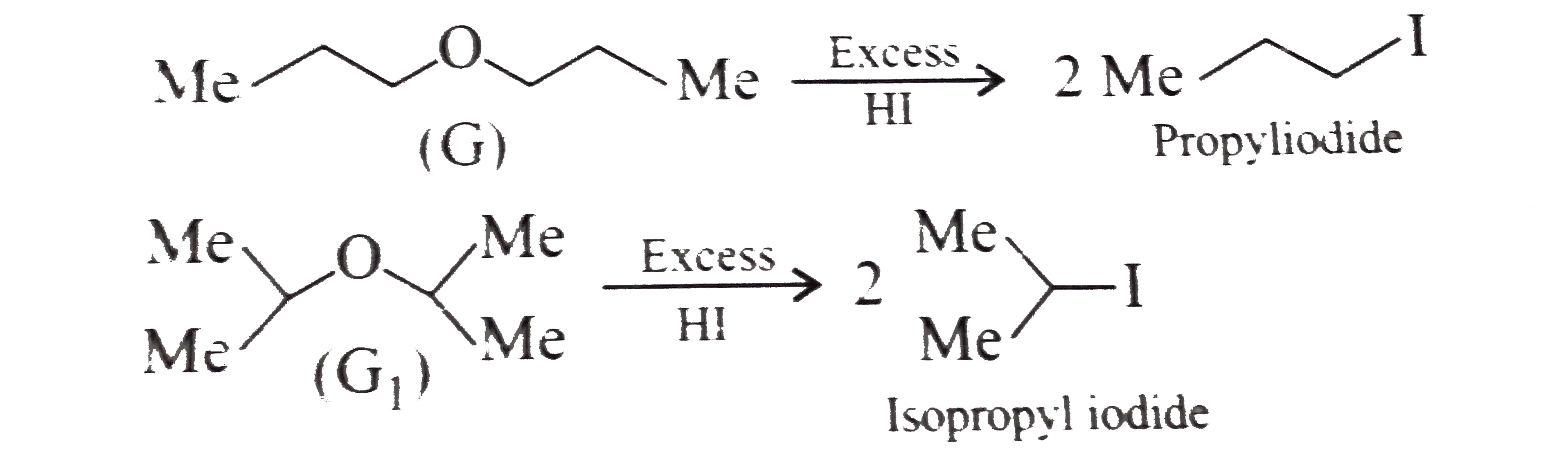Text Solution
Verified by Experts
Topper's Solved these Questions
ALCOHOL,PHENOL AND ETHERS
CENGAGE CHEMISTRY ENGLISH|Exercise Exercises Subjective|25 VideosALCOHOL,PHENOL AND ETHERS
CENGAGE CHEMISTRY ENGLISH|Exercise Exercises Concept Application|33 VideosALCOHOL,PHENOL AND ETHERS
CENGAGE CHEMISTRY ENGLISH|Exercise Archives Analytical And Descriptive|15 VideosALIPHATIC AND AROMATIC ALDEHYDES AND KETONES
CENGAGE CHEMISTRY ENGLISH|Exercise Archives Subjective|26 Videos
Similar Questions
Explore conceptually related problems
CENGAGE CHEMISTRY ENGLISH-ALCOHOL,PHENOL AND ETHERS-Solved Examples
- Explain why in the synthesis of ether (B) using (A) or A(1) all the th...
Text Solution
|
- Complete the following reactions: 2Me(3)C--Br overset (Ag(2)CO(3)) u...
Text Solution
|
- complete the following reactions: a. overset (C(6)H(6) + MeCOCI) un...
Text Solution
|
- a. Calculate the depression in freezing point (Delta T(f)) of 0.1 m s...
Text Solution
|
- Complete the following reactions :
Text Solution
|
- Synthesies the following alcohols by using: a. Grignard reagent (G....
Text Solution
|
- Write the reaction of EtOH with (i) KNH(2) (ii) aq. KOH (iii) Potassi...
Text Solution
|
- Identity the following compounds : Saturated ether with fewest C at...
Text Solution
|
- Given the stereochemical products B, C, and D of the following reactio...
Text Solution
|
- Give the product of the following reactions: a.
Text Solution
|
- a. How many conformational isomers are possible of 4-isopropyl cylohe...
Text Solution
|
- Explain which of the following reactions will occur. a.overset (RCOO...
Text Solution
|
- a. Which is a stronger nucleophile ? C(2)H(5)SH (ethane thiol or ethy...
Text Solution
|
- Distinguish between the following pairs:
Text Solution
|
- Distinguish between the following pairs: a. (I) PhOH (II) PhNH(2...
Text Solution
|
- NA (nucleophilic addition) reaction of alcohols with aldehydes gives h...
Text Solution
|
- Give the decreasing order of stability of the following quinones: a....
Text Solution
|
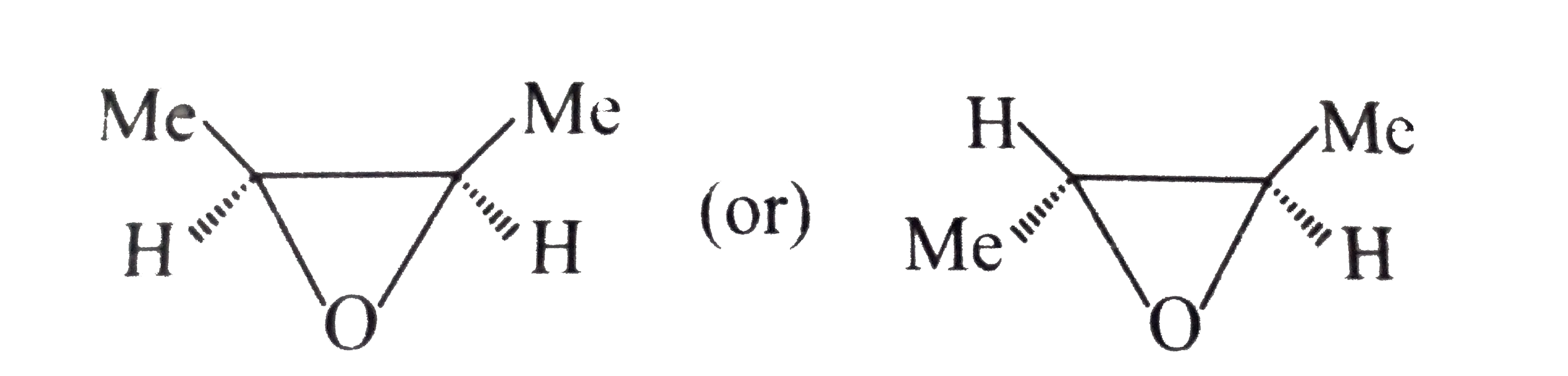 or
or 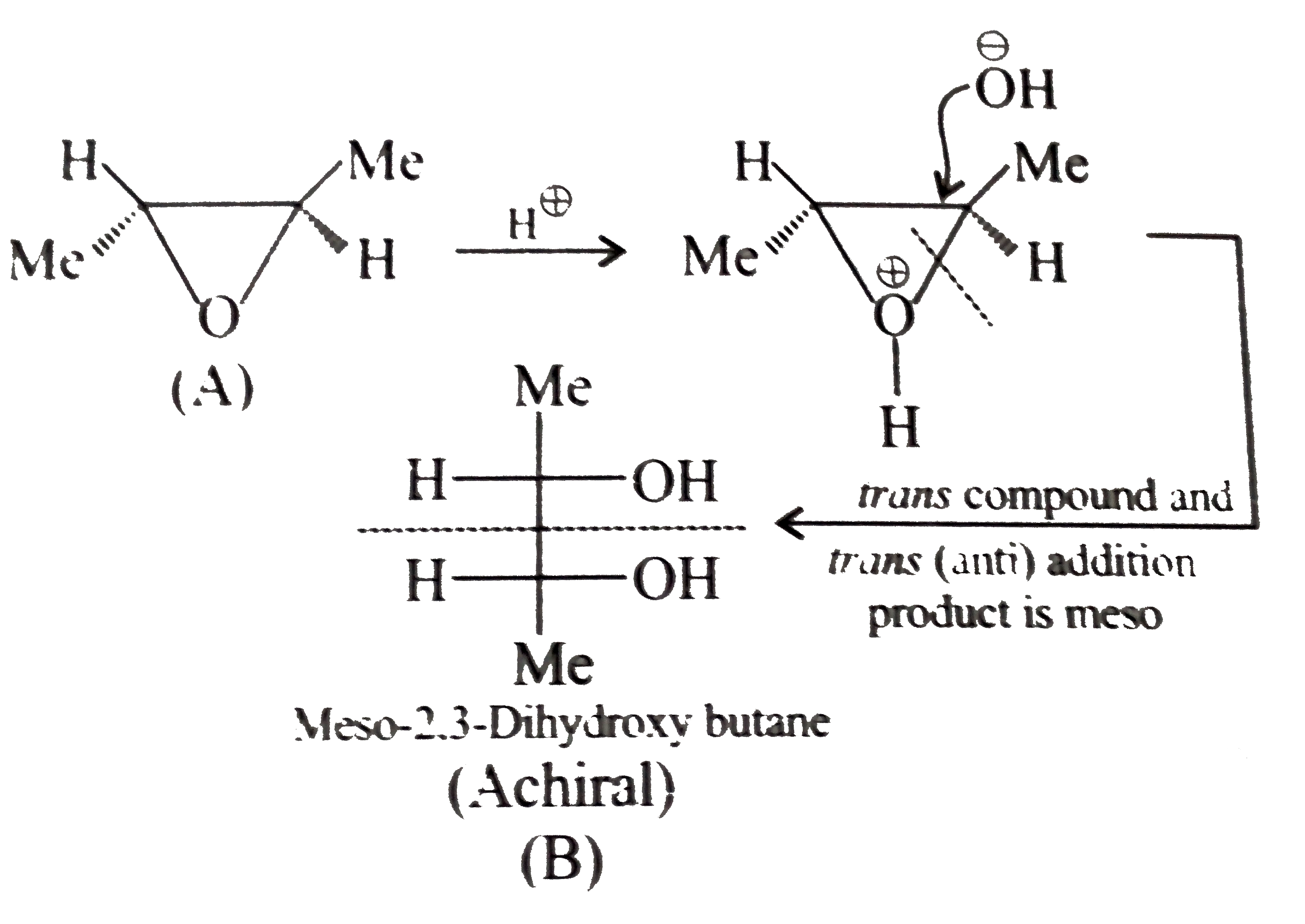
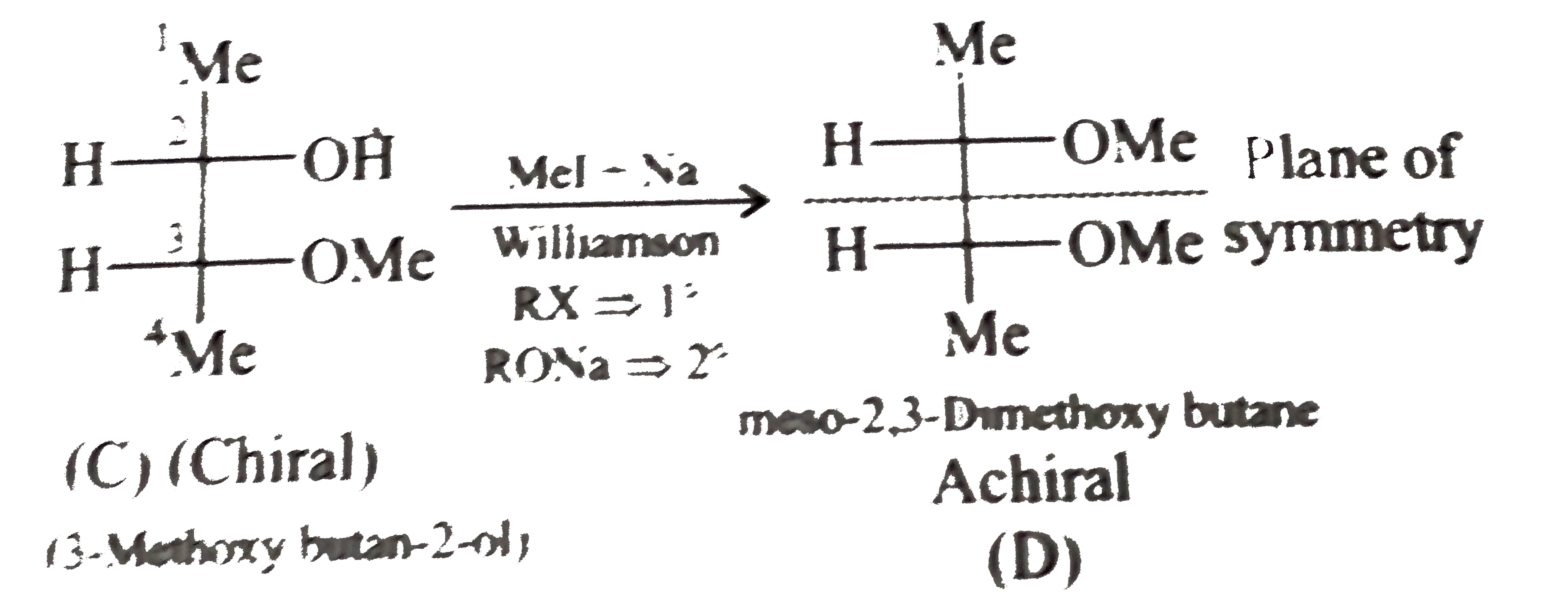
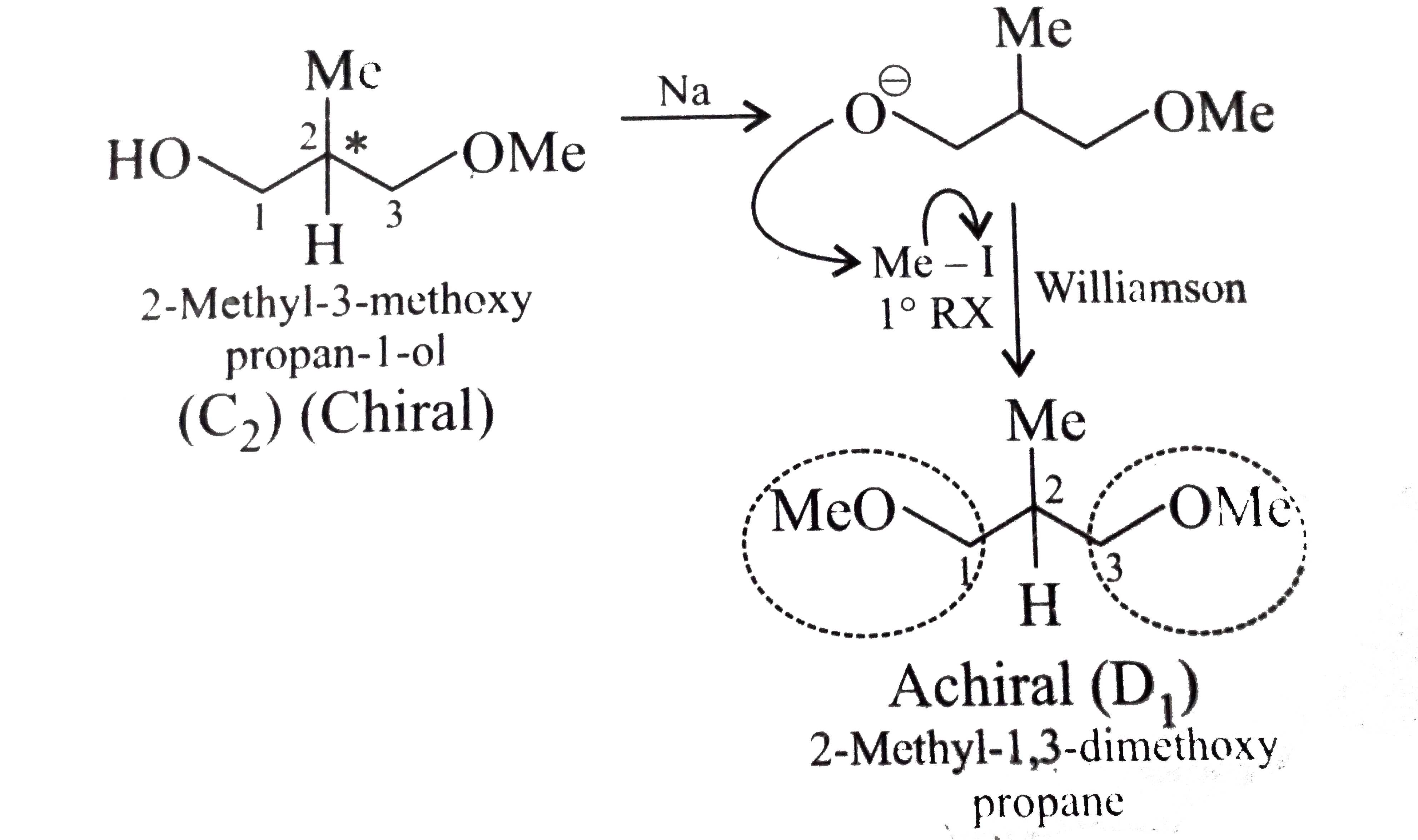
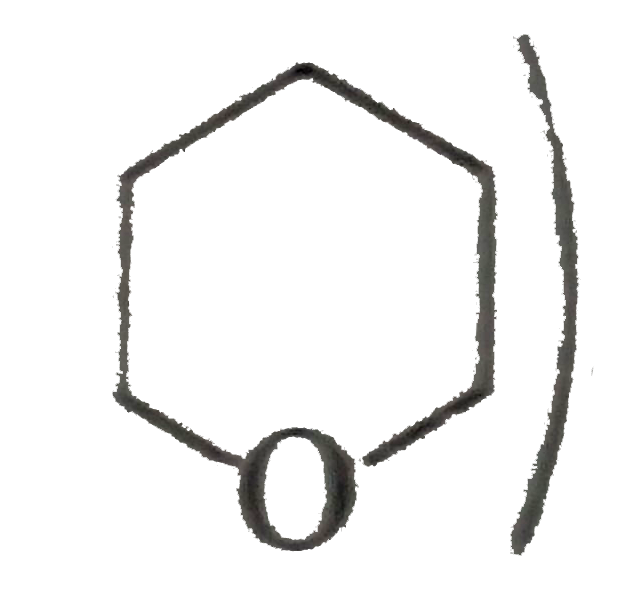
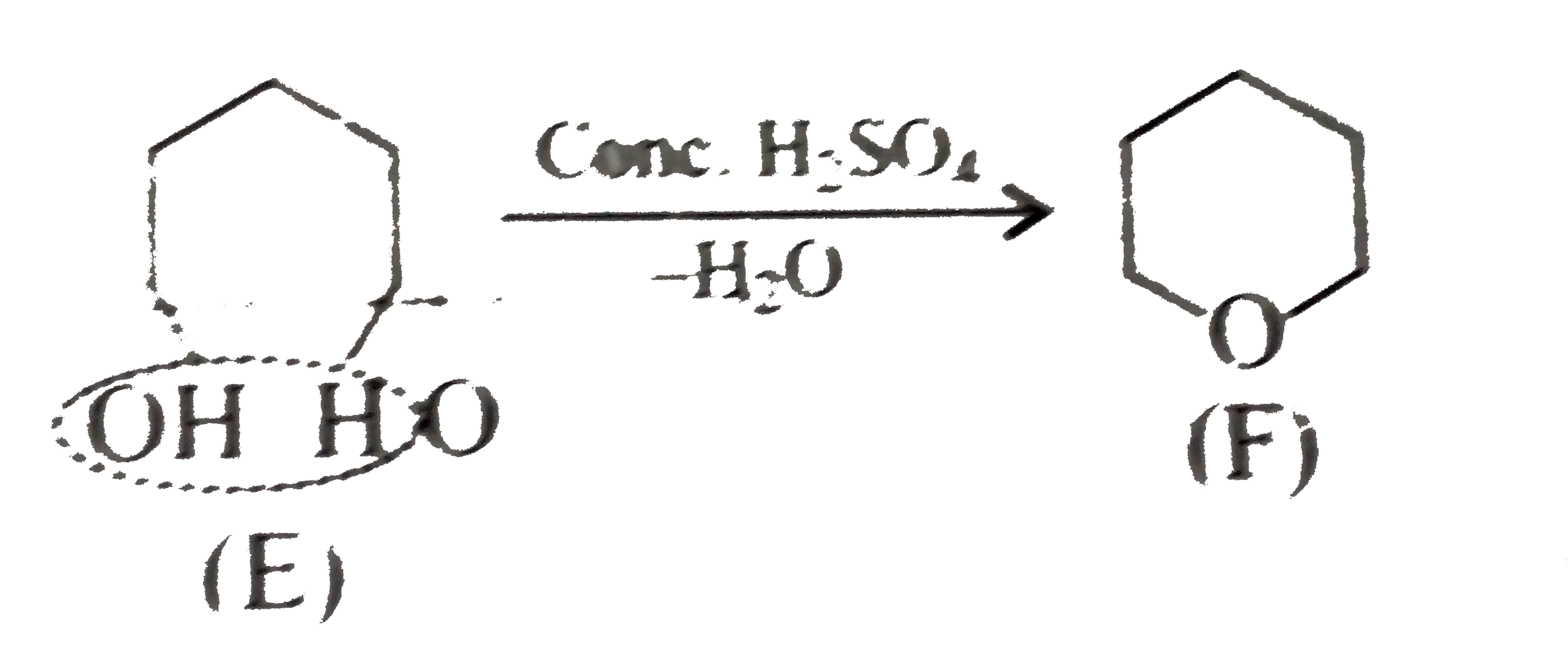 ) therefore (E) must be pentan 1,5-diol.
) therefore (E) must be pentan 1,5-diol. 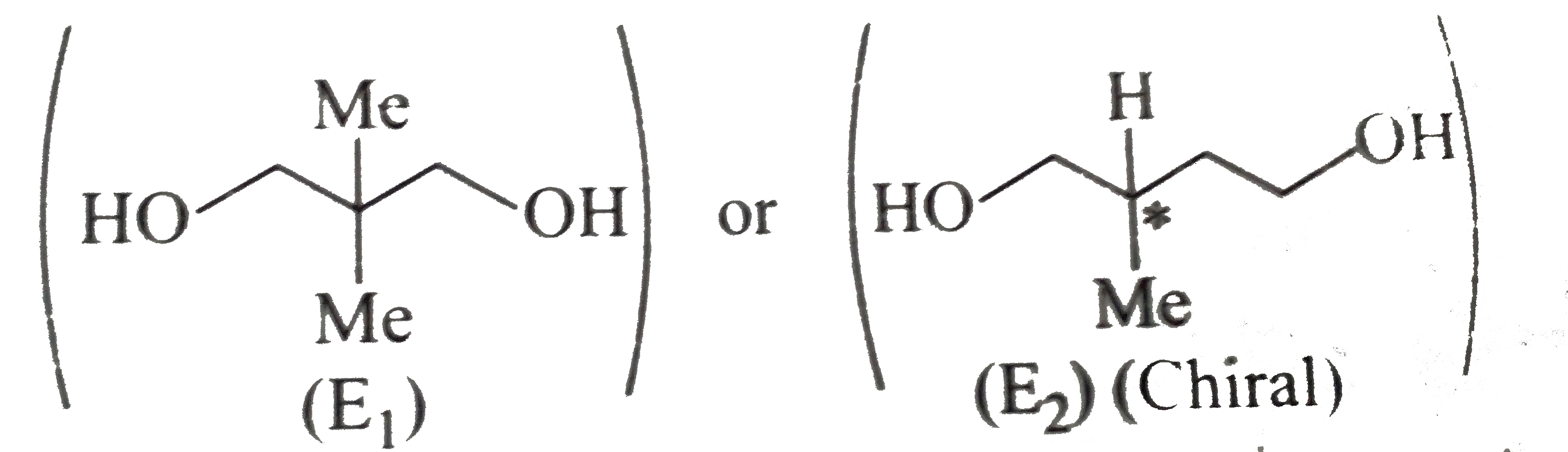
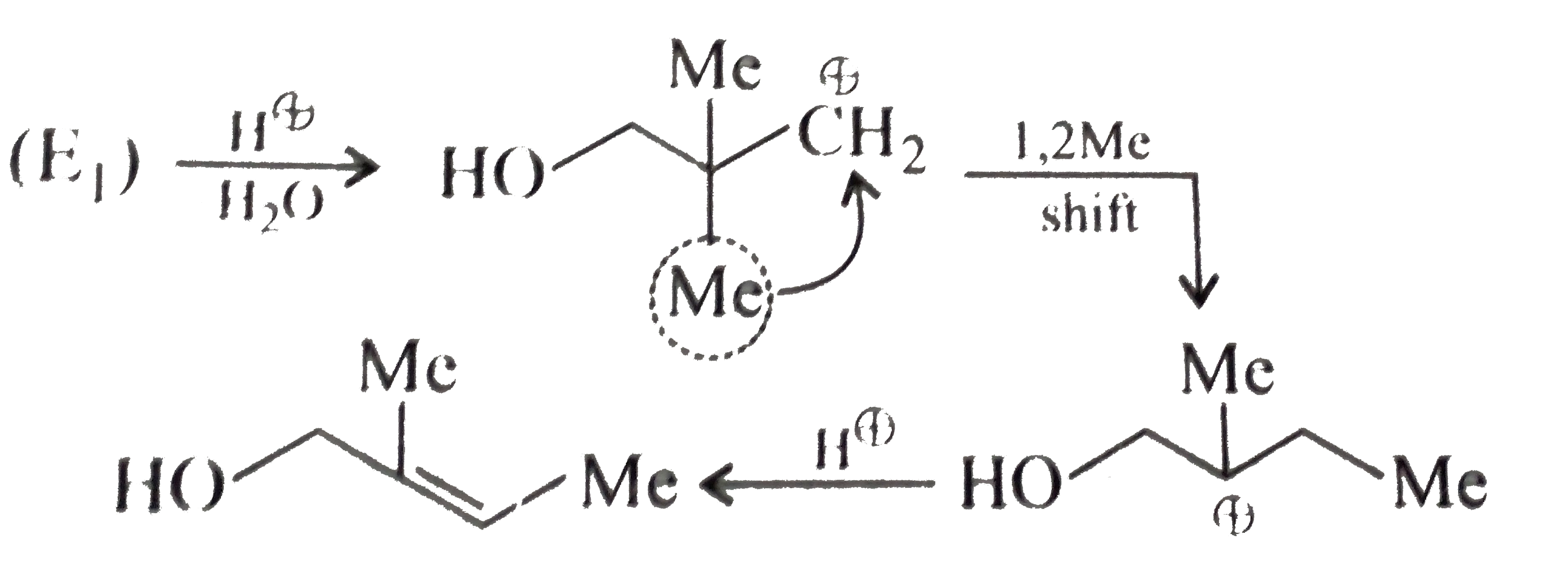 or
or