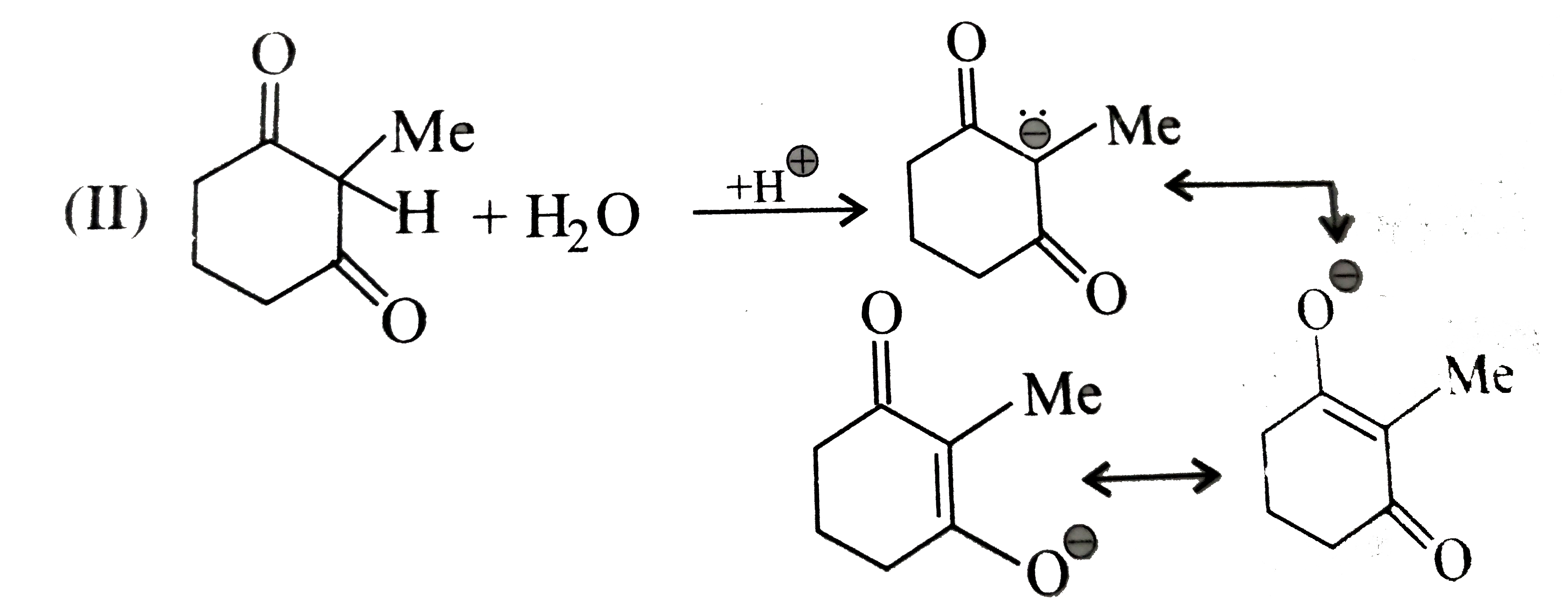
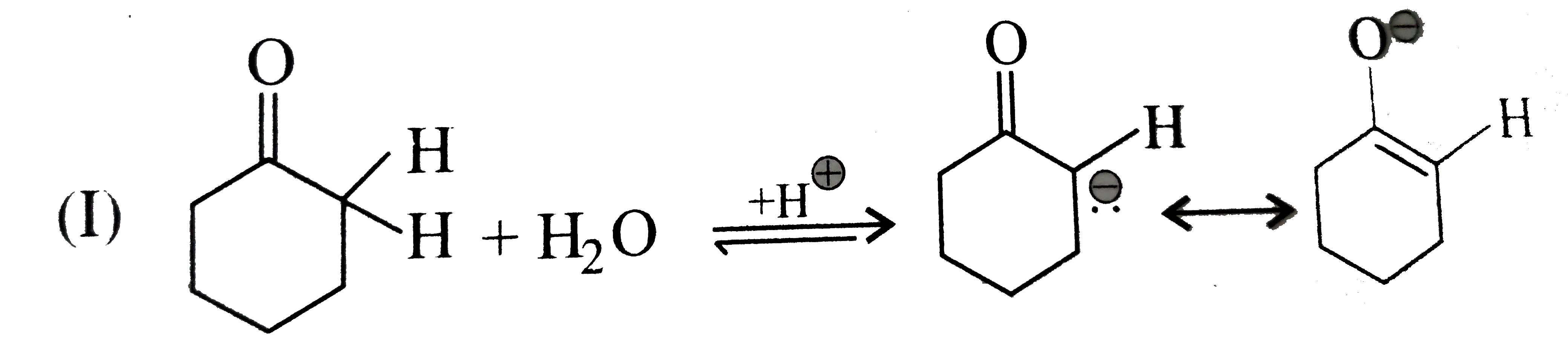
Text Solution
Verified by Experts
Topper's Solved these Questions
ALIPHATIC AND AROMATIC ALDEHYDES AND KETONES
CENGAGE CHEMISTRY ENGLISH|Exercise Solved Examples|13 VideosALIPHATIC AND AROMATIC ALDEHYDES AND KETONES
CENGAGE CHEMISTRY ENGLISH|Exercise Exercises Subjective|52 VideosALCOHOL,PHENOL AND ETHERS
CENGAGE CHEMISTRY ENGLISH|Exercise Archives Analytical And Descriptive|15 VideosAPPENDIX INORGANIC VOLUME 2
CENGAGE CHEMISTRY ENGLISH|Exercise Short Answer Type|179 Videos
Similar Questions
Explore conceptually related problems
CENGAGE CHEMISTRY ENGLISH-ALIPHATIC AND AROMATIC ALDEHYDES AND KETONES-Archives Subjective
- Explain: P-Aminobenzaldehyde (I) does not show nucleophilic addition...
Text Solution
|
- Write the structural formula of the main organic product formed when m...
Text Solution
|
- Outline the reaction sequence for the conversion of methanol to ethana...
Text Solution
|
- Show with balanced equation what happens when the following are mixed:...
Text Solution
|
- Write down the reactions involved in the preparation of the following ...
Text Solution
|
- Correct order of reactivity of CH(3)CHO,C(2)H(5)COCH(3)andCH(3)COCH(3)...
Text Solution
|
- Write down the main product of the following reaction : Propanal und...
Text Solution
|
- Give reason in one or two sentence for the following "Hydrazones of al...
Text Solution
|
- In what manner the following transformation might be carried out (in n...
Text Solution
|
- Complete the following with appropriate structures.
Text Solution
|
- Arrange the following in the increasing order of expected enol content...
Text Solution
|
- Write the structure of major organic product expected from the followi...
Text Solution
|
- How will you bring about the following conversion? "4-nitrobenzaldehyd...
Text Solution
|
- Complete the following reaction with appropriate structure. C(6)H(5)...
Text Solution
|
- Complete the following reaction with appropriate structure:
Text Solution
|
- Suggest appropriate structure of compound (A) (The number of carbon a...
Text Solution
|
- Draw the structure of 2-methylprop-1-ene
Text Solution
|
- Acetophenone on reaction with hydroxyl amine hydrochloride can produce...
Text Solution
|
- An aldehyde (A) (C(11)H(8)O), which does not undergo self aldol conden...
Text Solution
|
- Complete the following reactions with appropriate structures of produc...
Text Solution
|
- o-Hydroxy benzaldehyde is more soluble in water than p-hybroxy benzald...
Text Solution
|