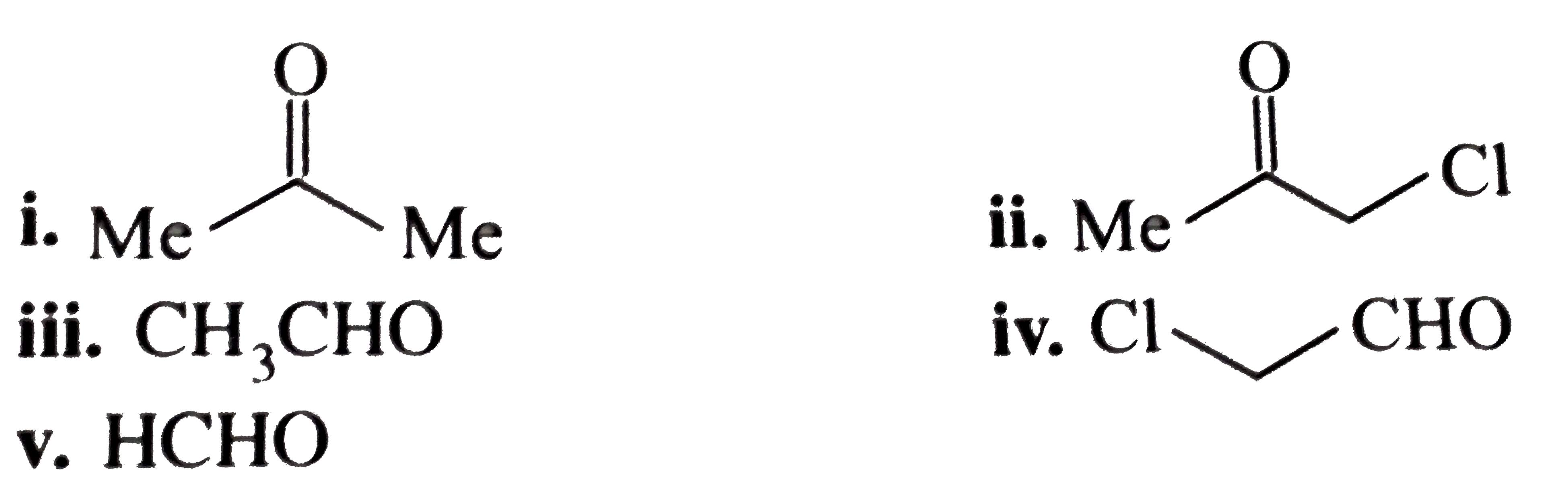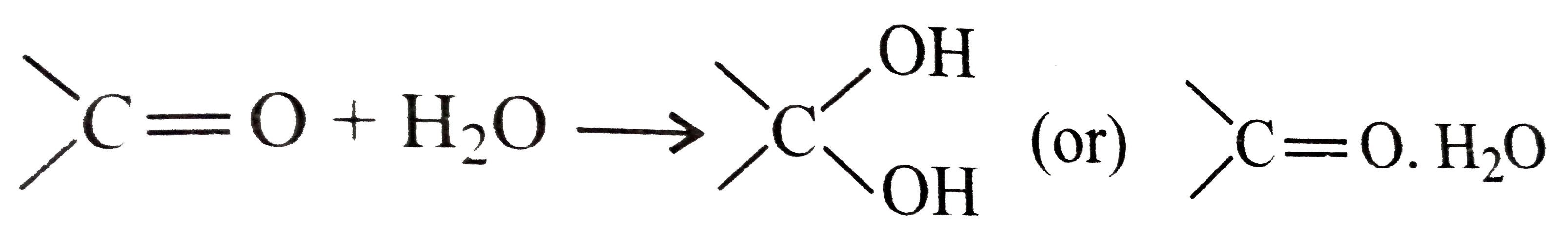Text Solution
Verified by Experts
Topper's Solved these Questions
ALIPHATIC AND AROMATIC ALDEHYDES AND KETONES
CENGAGE CHEMISTRY ENGLISH|Exercise Exercises Concept Application|20 VideosALIPHATIC AND AROMATIC ALDEHYDES AND KETONES
CENGAGE CHEMISTRY ENGLISH|Exercise Exercises Linked Comprehension|59 VideosALIPHATIC AND AROMATIC ALDEHYDES AND KETONES
CENGAGE CHEMISTRY ENGLISH|Exercise Solved Examples|13 VideosALCOHOL,PHENOL AND ETHERS
CENGAGE CHEMISTRY ENGLISH|Exercise Archives Analytical And Descriptive|15 VideosAPPENDIX INORGANIC VOLUME 2
CENGAGE CHEMISTRY ENGLISH|Exercise Short Answer Type|179 Videos
Similar Questions
Explore conceptually related problems
CENGAGE CHEMISTRY ENGLISH-ALIPHATIC AND AROMATIC ALDEHYDES AND KETONES-Exercises Subjective
- Two moles of HCHO and 1 mol of PhCHO react with conc. NaOH. What are t...
Text Solution
|
- Give the aldol condensation product of: a.
Text Solution
|
- Arrange the following compounds according to the extent of hydration. ...
Text Solution
|
- Convert CH(3)CHO into the following compounds: a. b.
Text Solution
|
- Identify: i. ii. iii. iv. v. vi.
Text Solution
|
Text Solution
|
Text Solution
|
- Complete the following: a. 2PhCHO+NH(2)NH(2)rarr b. Reduction of P...
Text Solution
|
- Complete the following: a.
Text Solution
|
- a. PhCOH(3) overset(NaOCI)underset(NaOH)rarr PhCOONa+CHCl(3) b. B...
Text Solution
|
- Convert salicyaldeyde into (coumarin).
Text Solution
|
- a.
Text Solution
|
- (B)overset(Θ)(OH)larr CH(2)=CH-CHO overset(AI(OEt)(3))rarr (A)
Text Solution
|
- p-Hydroxybenzaldehyde does not undergo Cannizzaro reaction. Explain.
Text Solution
|
- Complete the following reaction
Text Solution
|
- Convert HCHO to glycol in one step only.
Text Solution
|
- Convert and vice versa.
Text Solution
|
- Convert benzence to PhCHO in one step only.
Text Solution
|
- An aromatic kentone (X) has the molecular formula C(10)H(12)O(2). On v...
Text Solution
|
- A natural compound (A) (C(7)H(6)O) forms an oxime. When (A) is treated...
Text Solution
|