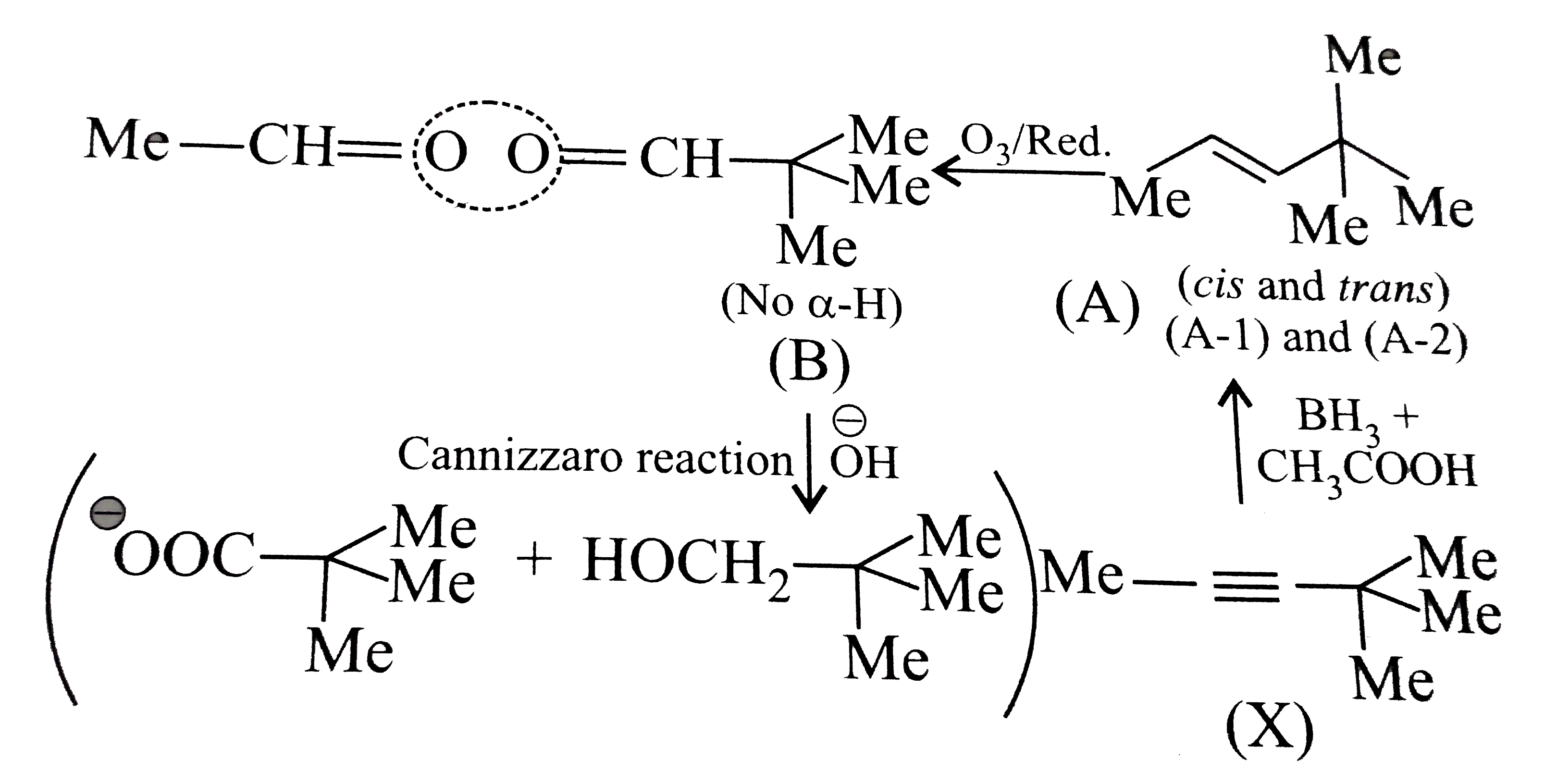Text Solution
Verified by Experts
Topper's Solved these Questions
ALIPHATIC AND AROMATIC ALDEHYDES AND KETONES
CENGAGE CHEMISTRY ENGLISH|Exercise Exercises Concept Application|20 VideosALIPHATIC AND AROMATIC ALDEHYDES AND KETONES
CENGAGE CHEMISTRY ENGLISH|Exercise Exercises Linked Comprehension|59 VideosALIPHATIC AND AROMATIC ALDEHYDES AND KETONES
CENGAGE CHEMISTRY ENGLISH|Exercise Solved Examples|13 VideosALCOHOL,PHENOL AND ETHERS
CENGAGE CHEMISTRY ENGLISH|Exercise Archives Analytical And Descriptive|15 VideosAPPENDIX INORGANIC VOLUME 2
CENGAGE CHEMISTRY ENGLISH|Exercise Short Answer Type|179 Videos
Similar Questions
Explore conceptually related problems
CENGAGE CHEMISTRY ENGLISH-ALIPHATIC AND AROMATIC ALDEHYDES AND KETONES-Exercises Subjective
- p-Hydroxybenzaldehyde does not undergo Cannizzaro reaction. Explain.
Text Solution
|
- Complete the following reaction
Text Solution
|
- Convert HCHO to glycol in one step only.
Text Solution
|
- Convert and vice versa.
Text Solution
|
- Convert benzence to PhCHO in one step only.
Text Solution
|
- An aromatic kentone (X) has the molecular formula C(10)H(12)O(2). On v...
Text Solution
|
- A natural compound (A) (C(7)H(6)O) forms an oxime. When (A) is treated...
Text Solution
|
- An organic compound (A) (C(8)H(14)O) forms an oxime and gives a positi...
Text Solution
|
- Compound (A) (C(6)H(12)O(2)) on reduction with LiAlH(4) yielded two co...
Text Solution
|
- Hydrocarbon (X), C(7)H(12), on reaction with boron hydride followed by...
Text Solution
|
- An organic compound (A) (C(6)H(10)) on reaction with CH(3)MgBr followe...
Text Solution
|
- An organic compound (A) contaning C, H, and O (16.32%) does not decolo...
Text Solution
|
- A 0.535 gm mixture of ethanol and acetaldehyde when heated with Fehlin...
Text Solution
|
- An organic compound (A) contains 79.25% C, 5.66% H, and 15.09% O. It h...
Text Solution
|
- An organic compound (A) containing 60.12% (C ) and 13.3% H has vapour ...
Text Solution
|
- Two organic compounds (A) and (B) containing 62.01% C and 10.3% H reac...
Text Solution
|
- An organic compound (A) (C(5)H(7)OCl) reacts rapidly with ethanol to g...
Text Solution
|
- An organic compound (A) contains 87.27% C and 13.73% H. Its vapour den...
Text Solution
|
- An organic compound (A) C(4)H(9)Cl on reacting with aqueous KOH gives ...
Text Solution
|
- An alkyne with five carbon atoms per molecule when passed through dilu...
Text Solution
|