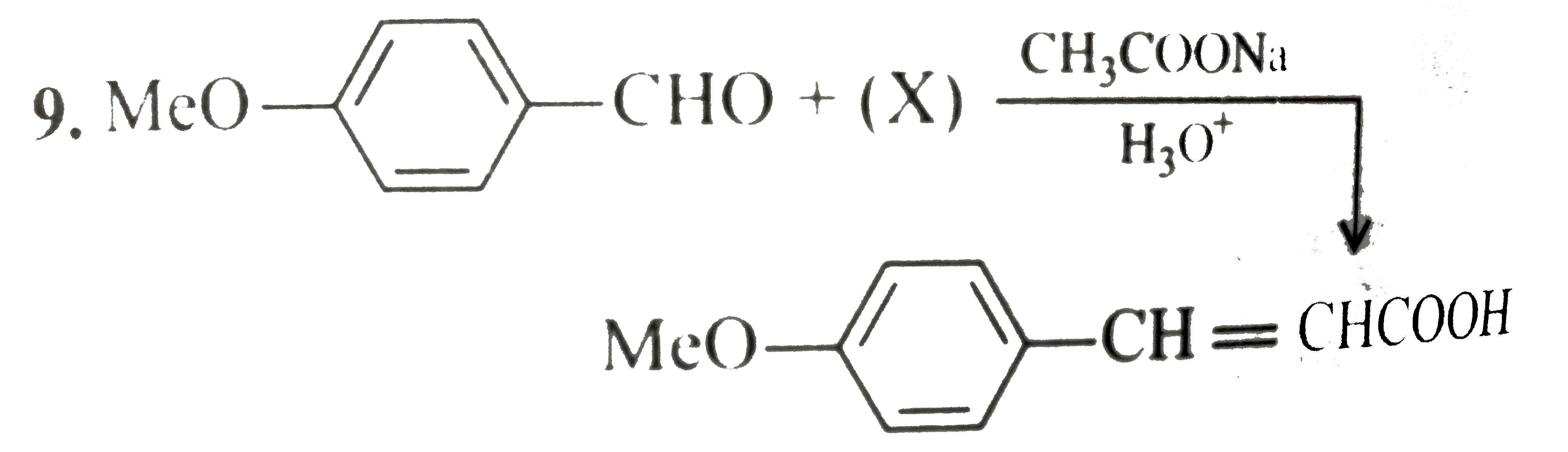A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
ALIPHATIC AND AROMATIC ALDEHYDES AND KETONES
CENGAGE CHEMISTRY ENGLISH|Exercise Archives Multiple Correct|8 VideosALIPHATIC AND AROMATIC ALDEHYDES AND KETONES
CENGAGE CHEMISTRY ENGLISH|Exercise Archives Fill In Theblanks|2 VideosALIPHATIC AND AROMATIC ALDEHYDES AND KETONES
CENGAGE CHEMISTRY ENGLISH|Exercise Exercises Assertion-Reasoning|6 VideosALCOHOL,PHENOL AND ETHERS
CENGAGE CHEMISTRY ENGLISH|Exercise Archives Analytical And Descriptive|15 VideosAPPENDIX INORGANIC VOLUME 2
CENGAGE CHEMISTRY ENGLISH|Exercise Short Answer Type|179 Videos
Similar Questions
Explore conceptually related problems
CENGAGE CHEMISTRY ENGLISH-ALIPHATIC AND AROMATIC ALDEHYDES AND KETONES-Archives Objective
- The Cannizzaro's reaction is not give by:
Text Solution
|
- m-Chlorobenzaldehyde on reaction with concentrated KOH at room temera...
Text Solution
|
- In Cannizzaro reaction given below 2 Ph CHO overset((-))overset(OH)r...
Text Solution
|
- Cl(3)C.CHO will reacts with water.True/False
Text Solution
|
- The enol form acetone after treatment with D(2)O gives:
Text Solution
|
- Which of the following has the most acidic hydrogen ?
Text Solution
|
- A mixture of benzaldehyde and formaldehyde on heating with aqueous NaO...
Text Solution
|
- overset((i)NaOH//100^(@)C)underset((ii)H^(+)//H(2)O)rarr The major p...
Text Solution
|
- Compound (X) is:
Text Solution
|
- In the following reaction sequence, the correct structures of (E ), (...
Text Solution
|