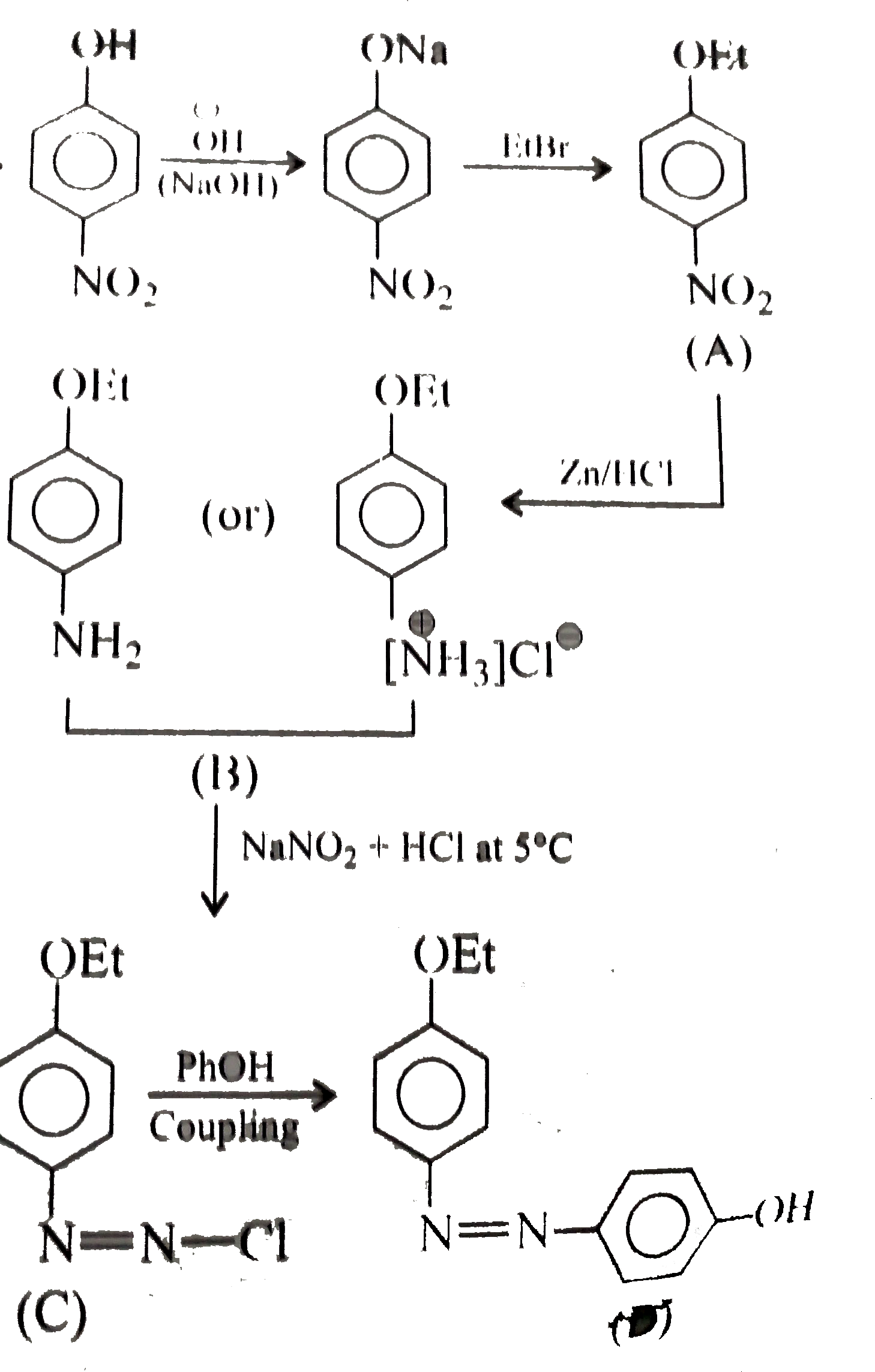
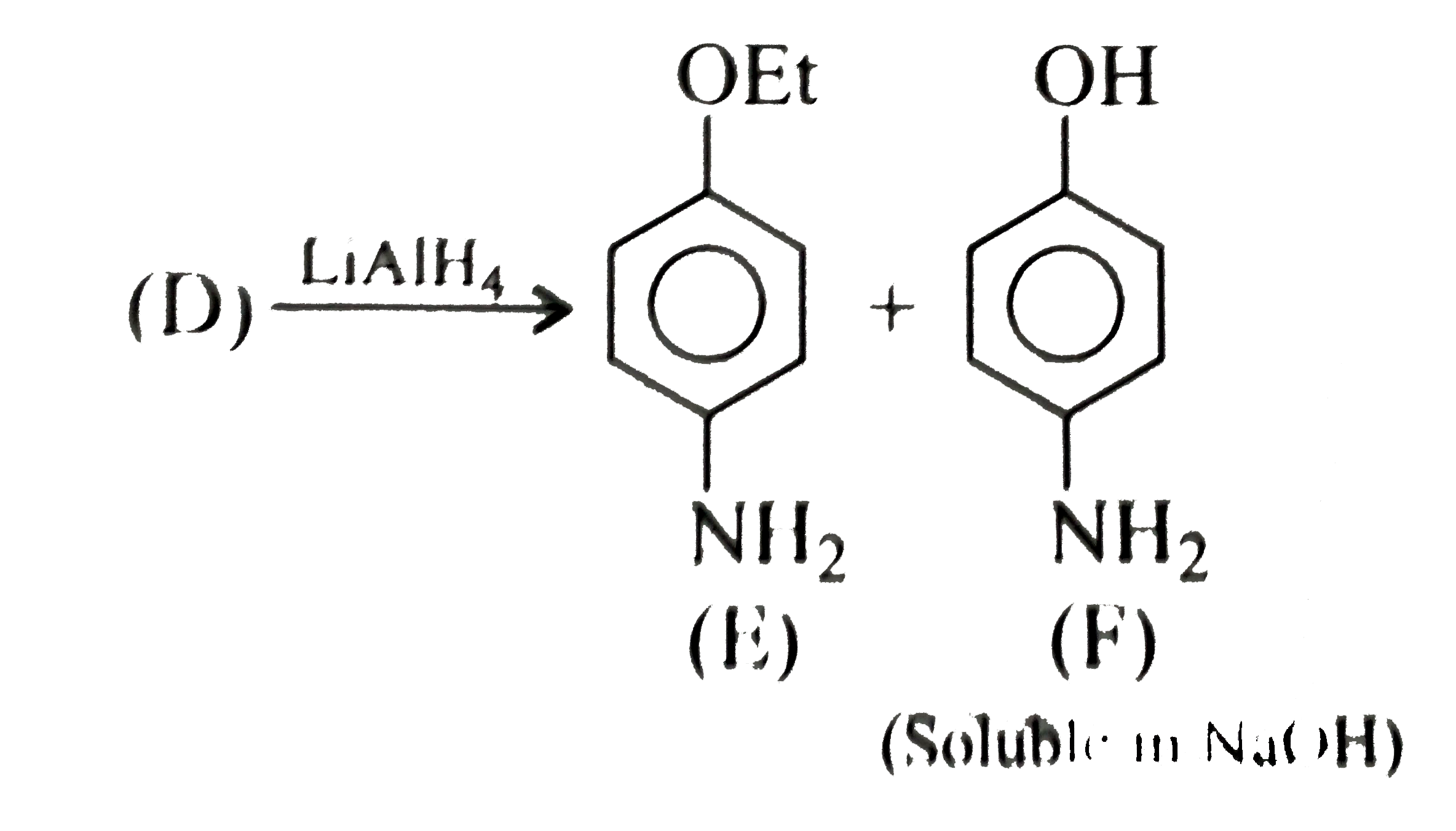
Text Solution
Verified by Experts
Topper's Solved these Questions
ORGANIC COMPOUNDS WITH FUNCTIONAL GROUP
CENGAGE CHEMISTRY ENGLISH|Exercise Exercises Subjective|21 VideosORGANIC COMPOUNDS WITH FUNCTIONAL GROUP
CENGAGE CHEMISTRY ENGLISH|Exercise Exercises Concept Application|13 VideosORGANIC COMPOUNDS WITH FUNCTIONAL GROUP
CENGAGE CHEMISTRY ENGLISH|Exercise Archives Analytical And Descriptive|24 VideosNUCLEAR CHEMISTRY
CENGAGE CHEMISTRY ENGLISH|Exercise Archives Subjective|13 VideosP-BLOCK GROUP 15 ELEMENTS - THE NITROGEN FAMILY
CENGAGE CHEMISTRY ENGLISH|Exercise Exercises Archives (Subjective)|28 Videos
Similar Questions
Explore conceptually related problems
CENGAGE CHEMISTRY ENGLISH-ORGANIC COMPOUNDS WITH FUNCTIONAL GROUP-Solved Examples
- Mention the main compounds which constitute Portland cement.
Text Solution
|
- Complete the following reactions. a. PhNO(2) overset(Zn+aq.NH(4)CI)r...
Text Solution
|
- Give two uses of (i) caustic soda (ii) quick lime
Text Solution
|
- Why is beryllium carbonate unusually unstable thermally as compared to...
Text Solution
|
- Explain the formation of the mixture PhCH(2)CHO (I) and PhCOMe (II) wh...
Text Solution
|
- What are the limitations of Hinsberg reagent?
Text Solution
|
- Why are lithium compounds soluble in organic solvents?
Text Solution
|
- Why metals like potassium and sodium can not be extracted by reduction...
Text Solution
|
- The negative electron gain enthalpy of fluorine is less than that of c...
Text Solution
|
- Write chemical name & formulae of a) Chile saltpetre b) Indian saltp...
Text Solution
|
- At what concentration ozone is harmful?
Text Solution
|
- A mixture of two organic compound is added to cold water. After filtra...
Text Solution
|