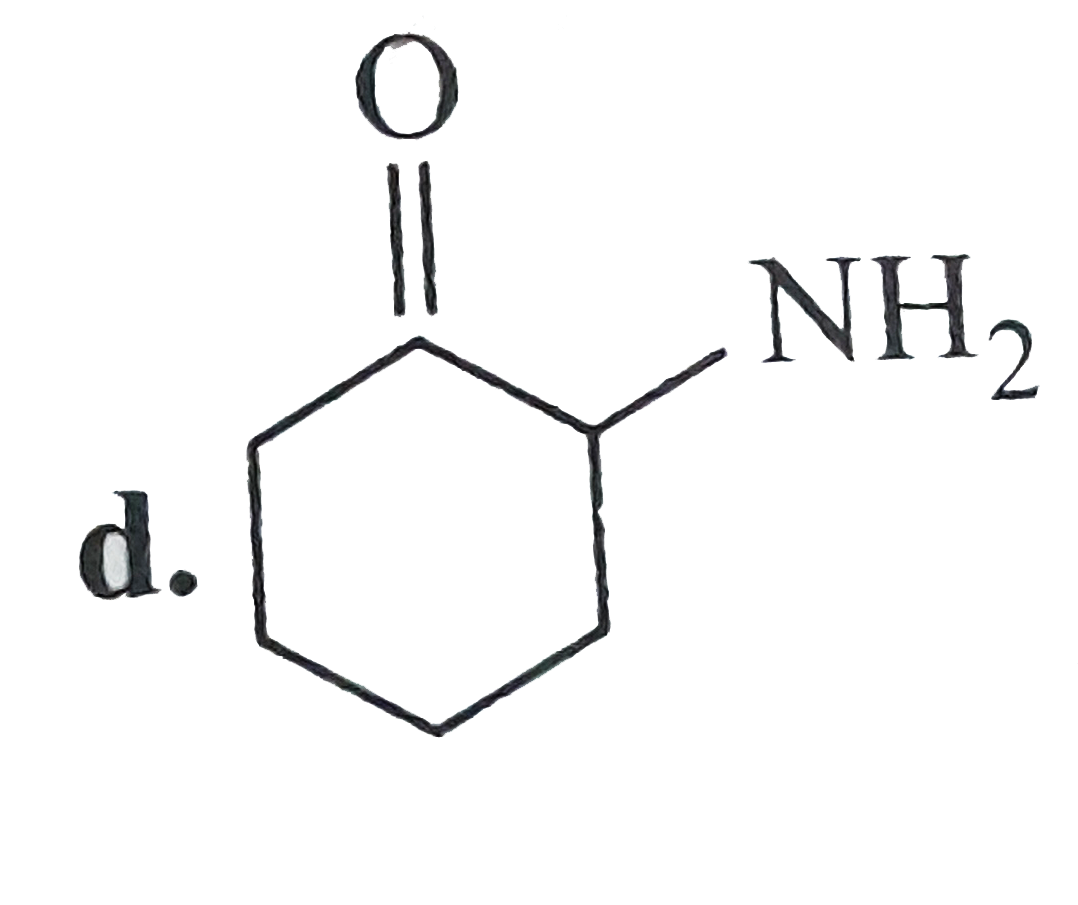
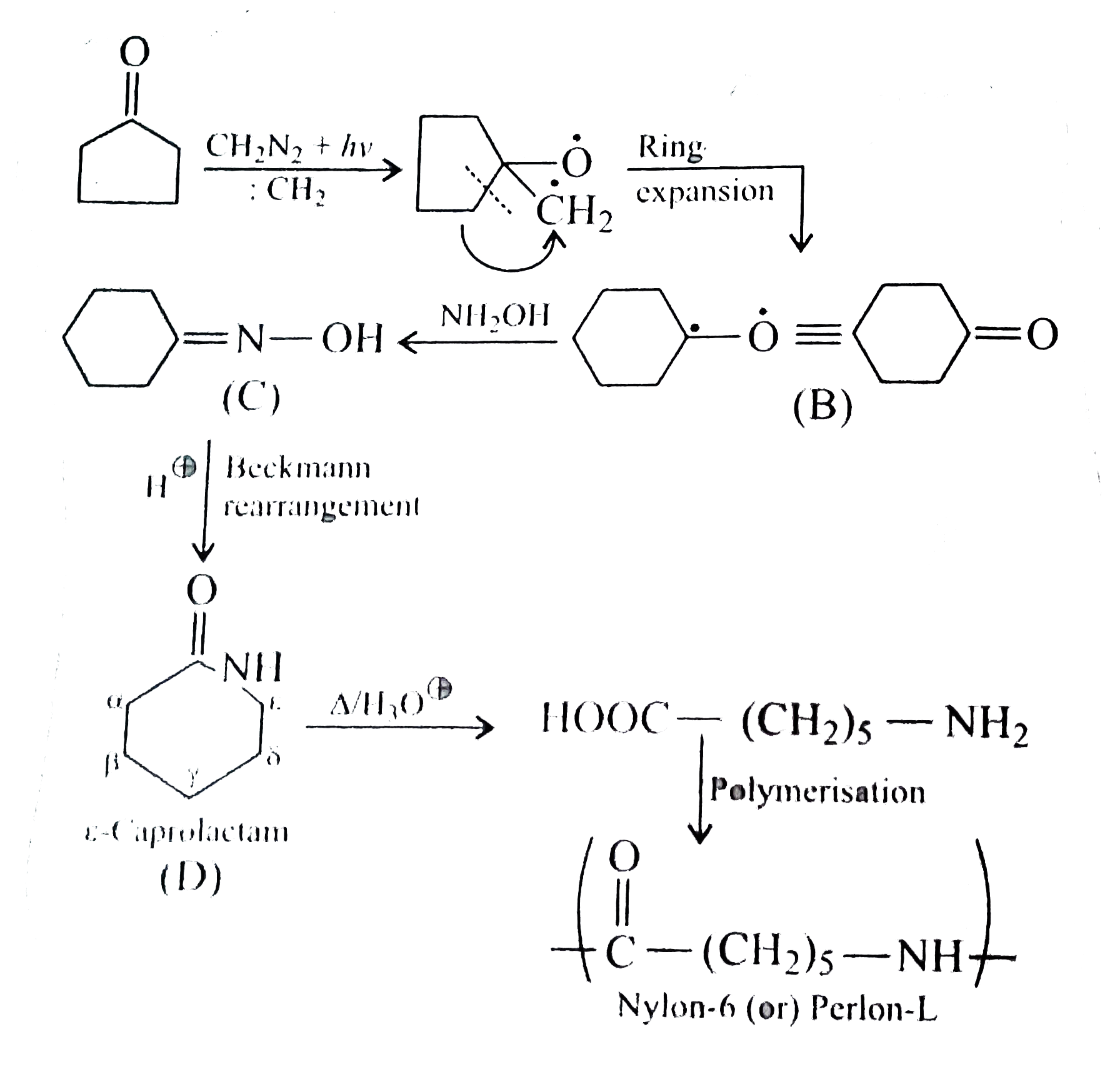
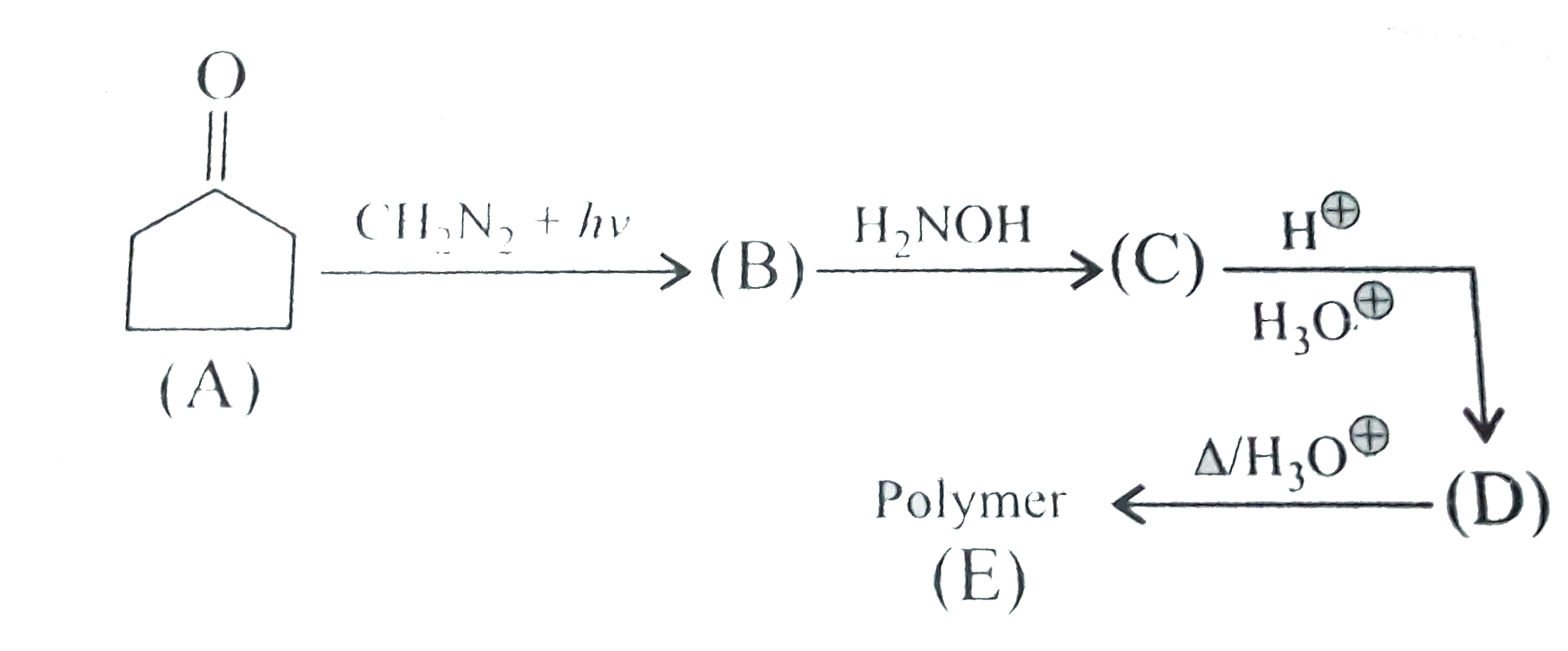A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
SYNTHETIC AND NATURAL POLYMERS
CENGAGE CHEMISTRY ENGLISH|Exercise Exercises Multiple Correct|28 VideosSYNTHETIC AND NATURAL POLYMERS
CENGAGE CHEMISTRY ENGLISH|Exercise Exercises Single Correct|94 VideosSYNTHETIC AND NATURAL POLYMERS
CENGAGE CHEMISTRY ENGLISH|Exercise Exercises Concept Application|20 VideosSURFACE CHEMISTRY
CENGAGE CHEMISTRY ENGLISH|Exercise Archives Subjective|2 Videos
Similar Questions
Explore conceptually related problems
CENGAGE CHEMISTRY ENGLISH-SYNTHETIC AND NATURAL POLYMERS-Exercises Linked Comprehension
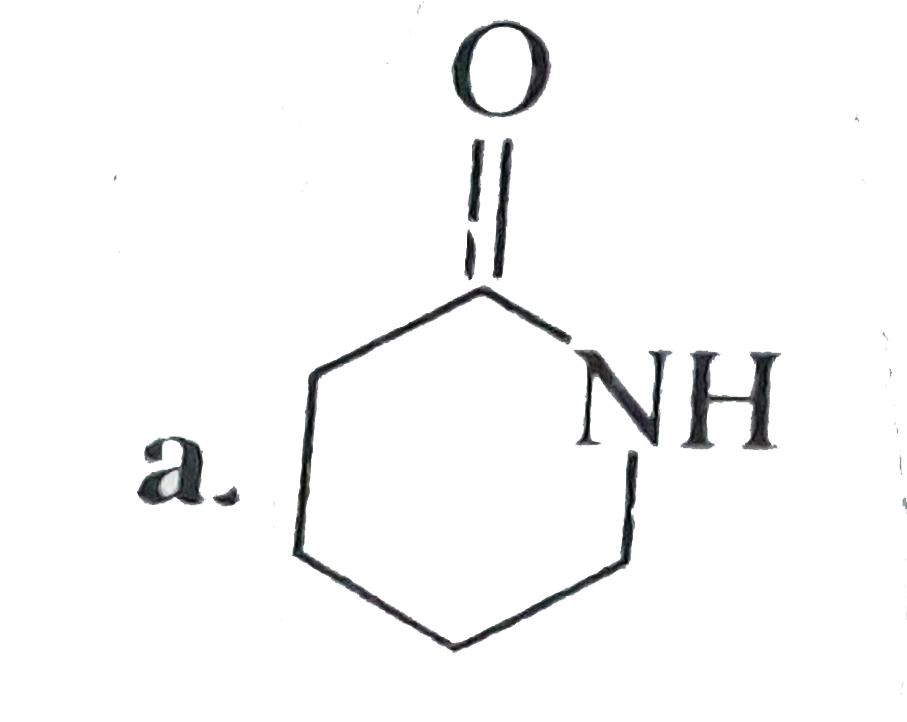
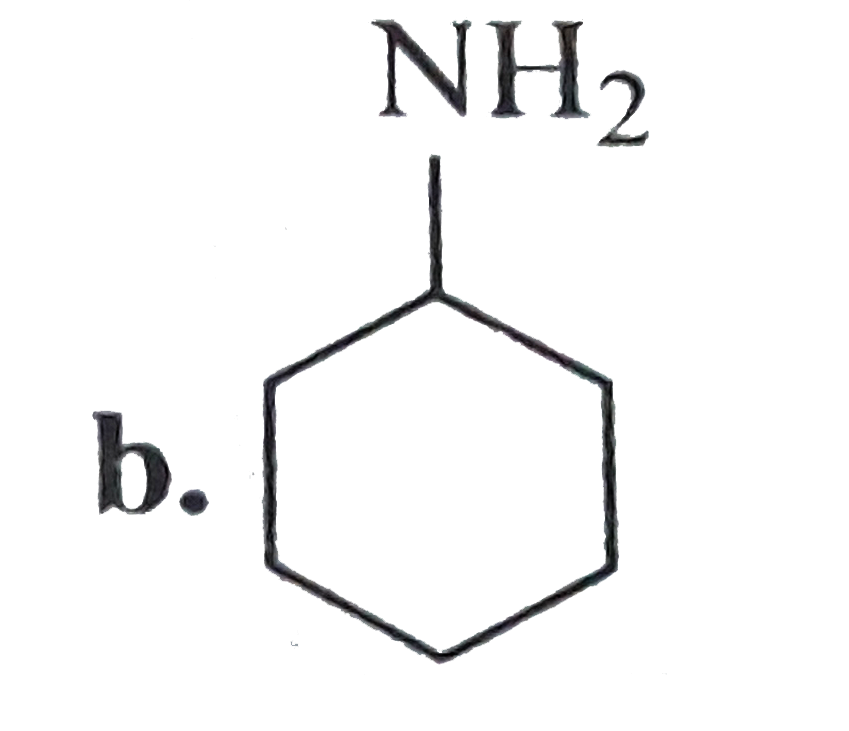
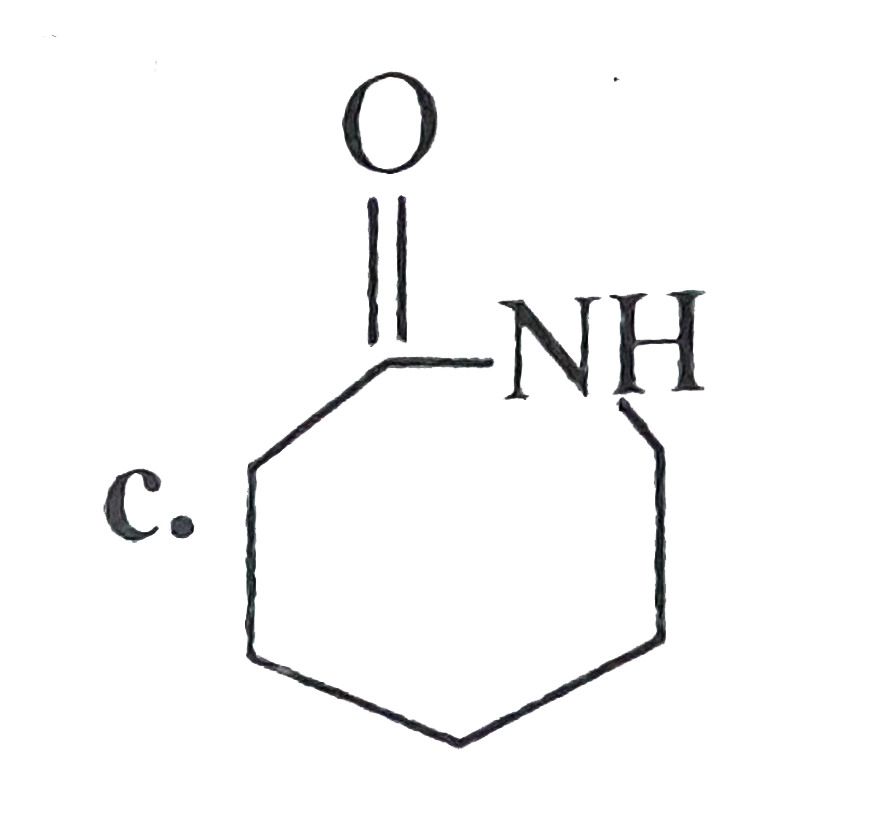
- Compund (B) is:
Text Solution
|
- Compound(C ):
Text Solution
|
- Compound (D)is:
Text Solution
|
- Compound (E)is:
Text Solution
|
- Compund (B) is:
Text Solution
|
- Compound(C ):
Text Solution
|
- Compound (D)is:
Text Solution
|
- Compound (E)is:
Text Solution
|
- Compound (F) is:
Text Solution
|
- Which of the following group does polymer(F) contains?
Text Solution
|
- Compound (C ) and(D) are:
Text Solution
|
- The linear polymer(E)is:
Text Solution
|
- The cross-linked polymer(F)is:
Text Solution
|
- The linear polymer(E)is formed is:
Text Solution
|
- Which of th following statement is/are correct about the polymer(E)?
Text Solution
|
- Polymer(E)is:
Text Solution
|
- Polymer(G)is:
Text Solution
|
- Which of the following groups does polymer(E)contain?
Text Solution
|
- Which of the following grpups polymer (G)contain?
Text Solution
|
- The conversion (A)(B)is called?
Text Solution
|